Liquid water evaporating into the gas phase is an example of this type of change
Physical Change
The addition of vinegar to baking soda causes the formation of bubbles. The indicator that a chemical reaction or change has occurred is
The formation of bubbles
2C8H18(l) + 25O2(g) → 18H2O(g) + 16CO2(g)
The classification for the above reaction
Combustion
5 g of hydrogen gas and 10 g of oxygen gas to form this amount of water
15 g of water
This is how we conducted an experiment on vinegar and baking soda
1) Measured the mass of both the vinegar and the baking soda
2) Added the two together
3) Noted observations
A change to a substance that occurs by forming a new substance
Chemical Change
Bubbles, formation of a precipitate, release or absorption of heat, and a color change are examples of ________ that tell us a chemical reaction has occurred
Indicators
2 Mg (s) + O2 (g) → 2 MgO (s)
The classification for the above reaction
Synthesis or Combination
Nitrogen and hydrogen gas are required to make ammonia. If 5 g of hydrogen gas are used to make 12 g of ammonia, this amount of nitrogen is required for the reaction
7 g of nitrogen
You are planning an investigation of the physical and chemical properties of sodium (Na) and water. You do a little research and find that a reaction occurs between the two in the reaction seen below.
Na (s) + H2O (l) → NaOH (aq) + H2 (g) + heat
These are the tools you would use for your experiment
A beaker for the water, tweezers to pick up the Na, balance to measure the mass of the water and the Na, and safety goggles for eye protection
Model 1 CO2(l) → CO2(g)
Model 2 CO2 (g) → C (s) + O2(g)
Model 1 represents this kind of change
Physical Change
(going from a liquid to a gas is a phase change, and thus a physical change)
2 Na (s) + 2 HCl (aq) → 2 NaCl(aq) + H2 (g) + heat
This indicator can be observed to know that the above chemical reaction has taken place
Formation of bubbles due to the release of H2 gas
Feels hot to the touch due to the release of heat
PbO2 (s) → Pb (s) + O2 (g)
The classification for the above reaction
Decomposition
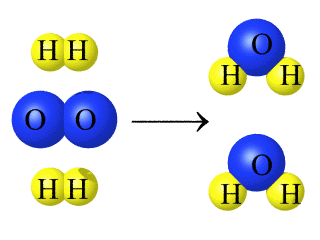
The above reaction obeys the law of conservation of matter because
The number of reactants and products are present in the same amount
You are planning an investigation of the physical and chemical properties of sodium (Na) and water. You do a little research and find that a reaction occurs between the two in the reaction seen below.
Na (s) + H2O (l) → NaOH (aq) + H2 (g) + heat
These are the steps you would use to carry out the experiment
1) Take the mass of the beaker
2) Add a certain amount of water to beaker. Record the mass
3) Place the Na on the balance. Record the mass
4) Place the Na in the beaker with the water. Record observations.
5) Record the new mass of the beaker
If you place a piece of a saltine cracker on your tongue for a few minutes, it begins to taste sweet. Do you think a chemical or physical change leads to this change in taste? Why?
Taste change would indicate that a new substance has formed, which indicates a chemical change
2C8H18(l) + 25O2(g) → 18H2O(g) + 16CO2(g) + heat
The above reaction is carried out in a large water jug. The indicators that tell us a chemical change has occurred are
The formation of flames as the reaction is a combustion reaction.
The collection of water that did not escape in the gas phase.
Smoke that is carbon dioxide or water vapor
2 Al (s) + 6 HCl (aq) → 2 AlCl3 (aq) + 3 H2 (g)
The classification for the above reaction
Single Displacement
Single Replacement
If 20 g of copper are needed to synthesis 32 g of CuO, this amount of oxygen is needed for the reaction
12 g of oxygen
You are planning an investigation of the physical and chemical properties of sodium (Na) and water. You do a little research and find that a reaction occurs between the two in the reaction seen below.
Na (s) + H2O (l) → NaOH (aq) + H2 (g) + heat
These are the indicators you would look for to know a chemical reaction occurred
The formation of H2 bubbles due to the release of gas
The beaker would feel hot due to the release of heat
Identify the change and explain your answer:
Black iron and yellow sulfur are heated and form a non-magnetic shiny grey substance.
Chemical change, a new substance with new properties was formed
AgNO3 (aq) + KCl (aq) → AgCl (s) + KNO3(aq)
The observed change to indicate that a chemical reaction has occurred is
The formation of the solid precipitate, AgCl (s)
Ca(OH)2 (s) + 2 HCl (aq) → CaCl2 (aq) + 2 H2O (l)
The classification for the above reaction
Double-Displacement
Double-Replacement
2 NaCl + MgF2 ---> 2 NaF + MgCl2
A company uses 10 g of NaCl and 22 g of MgF2 to make 13 g of NaF and this mass of MgCl2
19 g of MgCl2
You are planning an investigation of the physical and chemical properties of sodium (Na) and water. You do a little research and find that a reaction occurs between the two in the reaction seen below.
Na (s) + H2O (l) → NaOH (aq) + H2 (g) + heat
At the end of the experiment, you notice that the total mass after the reaction is complete is less than the initial mass. This observation can be explained by
The loss of H2 gas
The Mentos and Coke experiment is this type of change for this reason
The mentos is a rough candy and provides a nucleation point of the carbonation already dissolved in the Coke. This makes the mentos and Coke experiment a physical change.
Why do we use baking soda instead of starch to make cakes fluffy?
Starch does not react with water or acid to make bubbles. Bubbles are what make cakes fluffy. Baking, soda, however, will make bubbles
One pollutant produced in automobile engines is nitrogen dioxide (NO2). It changes to form nitrogen oxide (NO) and oxygen gas when exposed to sunlight. Of what reaction type is this an example?
This is a decomposition reaction
A thin strip of iron with a mass of 15.5g is placed into a solution containing 21.0g of copper (II) sulfate and copper begins to form. After some times, the reactions stops because all the copper (II) sulfate has reacted. The iron strip is found to have a mass of 8.5g. The mass of copper formed is found to be 8.60g. What mass of iron (II) sulfate has been formed in the reaction?
Mass of iron = Initial mass – Final mass
= 15.5 – 8.5 = 7.0g
Mass of copper (II) sulfate = 21.0g
Mass of copper = 8.60g
According to law of conservation of mass,
Mass of iron + Mass of copper (II) sulfate = Mass of copper + Mass of iron (II) sulfate
7.0g + 21.0g = 8.60g + Mass of iron (II) sulfate
So, Mass of iron (II) sulfate = 19.40g
Explain why it is best to store many chemicals in tightly sealed bottles in dark locations at cool or moderate temperatures
A tightly sealed bottle keeps reactive substances in air away from the chemicals so they cannot react with them. Storing chemicals at low temperatures slow down any possible reactions that may occur. The dark bottle prevents light from reacting the chemicals, which could also start up a reaction.