What is a cation and what is an anion?
cat- positive
an-negative
Al4 C3
Aluminum Carbide
What kind of bond is NaCl?
Ionic
Name the 5 types of reactions
double replacement, single replacement, combustion, synthesis, decomposition
Calculate the percent yield if the theoretical yield is 50.0 g of a product and the actual yield is 42.0 g.
84%
What is an ionic bond?
metal and non-metal bonded
Na2O
What kind of bond is in H2O?
Polar Covalent
Define exothermic rxn
An exothermic reaction is a chemical reaction in which less energy is needed to break bonds in the reactants than is released when new bonds form in the products.
calculate the molar mass of UF6
What is a covalent bond?
2 nonmetals bonded
Mn3 (PO4)2 The polyatomic ion has the formula PO3-4
Manganese(II) phosphate
What is a metallic bond?
Define Endothermic rxn
find the molar mass of (NH4)2SO4
132.1 g/mol
What is the most common bond of Noble gasses?
no bonds, they have 8 valence electrons
The older method uses the suffixes ___ and ___ to denote the lower and higher charges, respectively.
-ous, -ic
what kind of bonds are in polyatomic ions?
covalent bonds
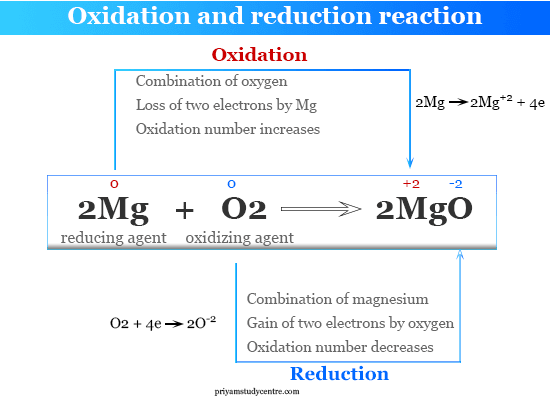
2Mg+O2->2Mg+2O-2
45.2g of Fe reacts with excess O2 to produce 58.1g of Fe3O. Given the unbalanced equation:
Fe + O2 ->Fe3O4
What is the Theoretical Yield?
69.10gFe3O4
How many significant figures are in the number 0.004030.
5
Double Jeopardy!
In the Old way, name:
CuCl2
CuCl
Cupric Chloride
Cuprous Chloride
What elements bond in a hydrogen bond?
N, O, or F
balance the equation and identify the reaction type
CaCl2+Na3PO4->Ca3(PO4)+NaCl
3CaCl2+2Na3PO4->Ca3(PO4)+6NaCl
50g of Fe reacts with excess O2 to produce 60g of Fe3O. Given the unbalanced equation:
Fe + O2 ->Fe3O4
What is the percent yield?
86.33%