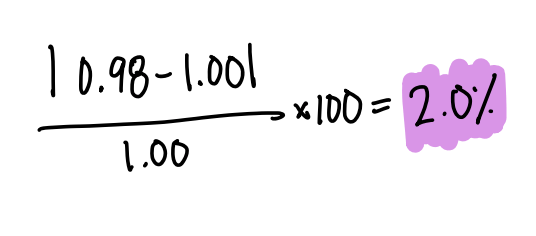All measurements taken with this rules would end in which place value and unit?

What is 100ths place and centimeters?
X.XX cm
120 g in kg
What is 0.12 kg?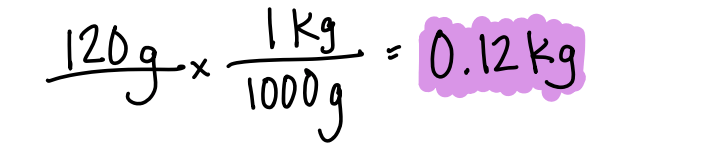
The number of sig figs in 0.02050 m
What is 4?
The volume of a 73.9 g sample of tin with a density of 7.21 g/cm3.
What is 10.2 cm3?
Number of sig figs in 56000 L
What is 2?
The place value and unit which this graduated cylinder reports volumes
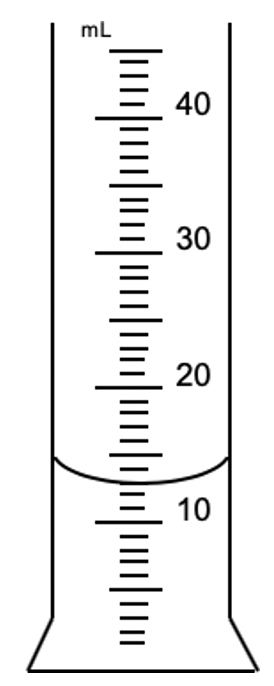
What is the 10ths place and milliliters?
13.0 mL
3.28 km in cm
What is 3.28 105 cm?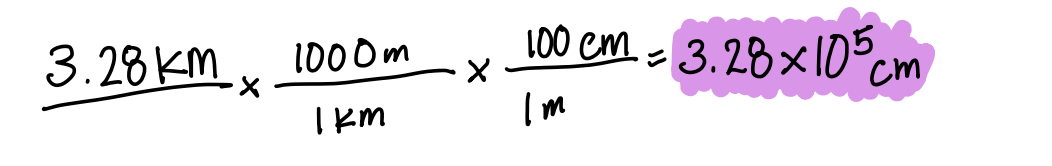
5.81 cm - 0.983 cm = ??
What is 4.83 cm?
The density of chromium is 7.19 g/cm3. The volume of a 0.31 kg sample of Cr.
What is 43 cm3?
Round 1864.2 km to 2 significant figures
1900 km
What is the length of the object below?
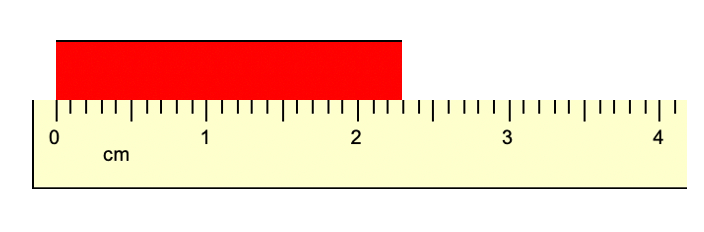
What is 2.29 cm?
or
What is 2.30 cm?
You are giving a patient 3.0 cm3/min of a given solution intravenously. If this is done for 24 hours, how many liters of this solution will you need?
4.3 L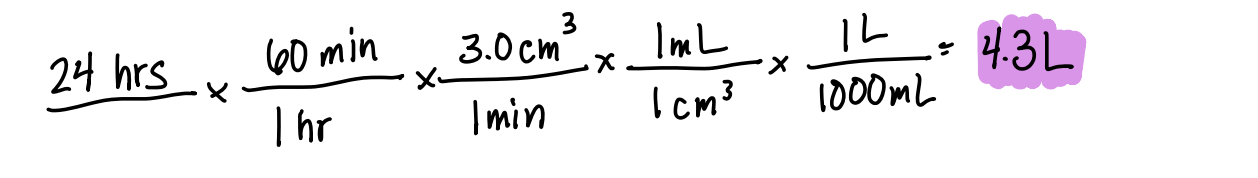
5.7621 m x 6.901 m x 0.460 m =
18.3 m3
A block of copper metal has a mass of 1896 g. The dimensions of the block are 8.40 cm x 5.45 cm x 4.6 cm. What is the density of copper?
9.0 g/cm3
What sport did Ms. Zibart play in high school?
Badminton
Determine the volume of the liquid in the graduated cylinder.
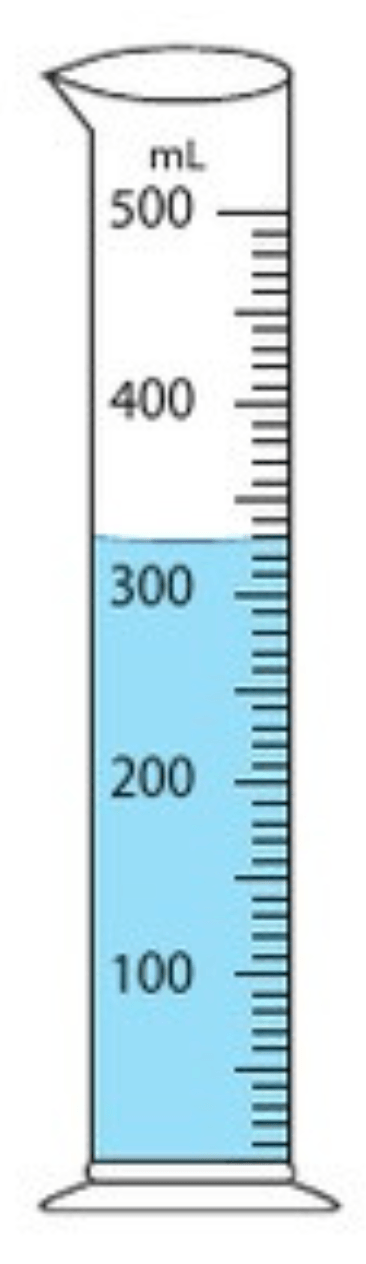
What is 328 mL?
(the range 326 - 330. would be acceptable)
You are buying carpet to cover a room that measures 39 ft by 40. ft. The carpet cost $18 per square yard. How much will the carpet cost?
$3100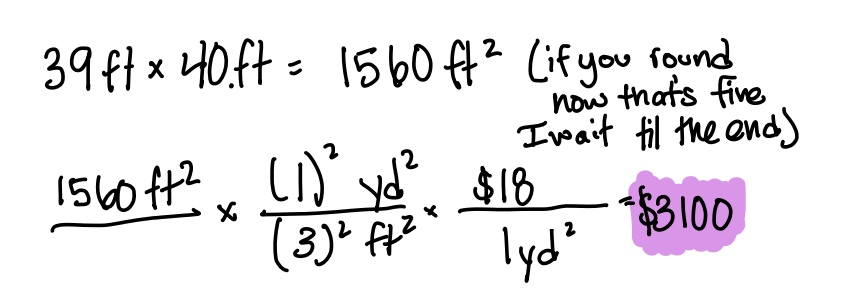
896.72 km + 1900 km = ??
What is 2800 km?
Aluminum can be sold to chemistry teachers in strips that are 5.00 cm wide and 0.40 cm thick. If a chemistry teacher needs a piece of aluminum that has a mass of 54.0 g, how long a piece would she need to cut? (density of aluminum 2.70 g/cm3)
l= 10. cm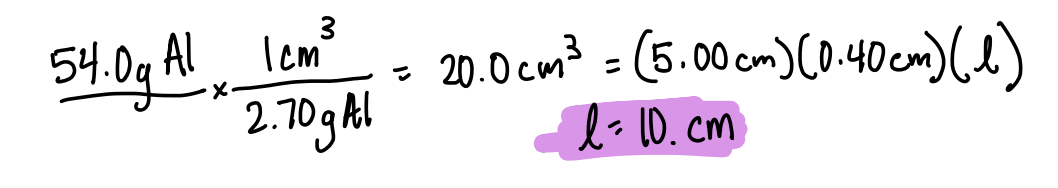
Four students measured the density of aluminum (2.70 g/cm3). Which student was accurate & precise? Which student was the neither accurate nor precise? Calculate John's % Error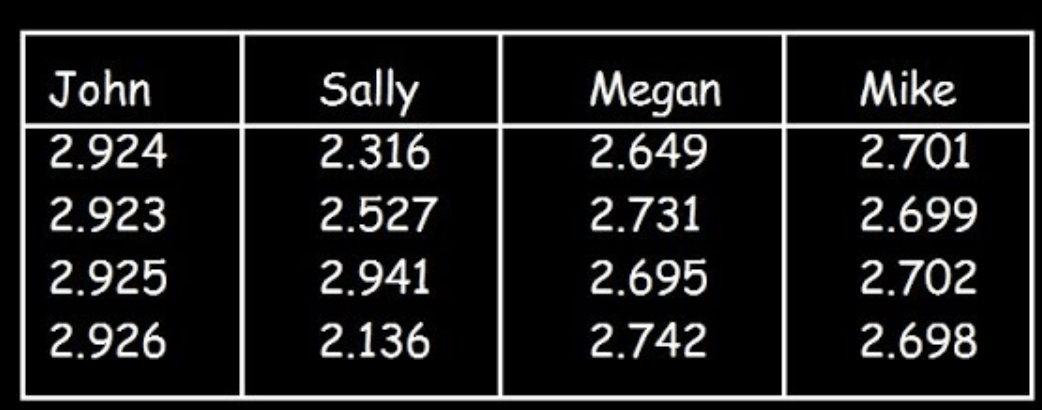
accurate & precise: Mike
neither accurate nor precise: Sally
John had 8.33% error
The temperature reading of the thermometer

What is 19°C?
(Since the thermometer increases by 2°C increments, can only be significant to the ones place)
A local zoning ordinance says that a house’s “footprint” (area of its ground floor) cannot occupy more than 25% of the lot it is built on. Suppose you own a 0.33 acre lot (1 acre = 43,560 ft2). What is the maximum allowed footprint for your house in square meters?
330 m2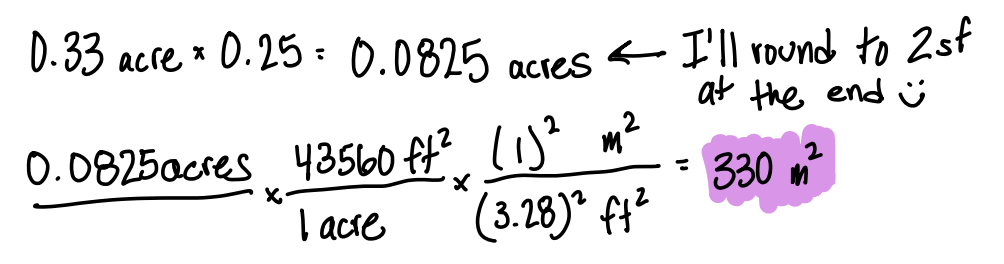
1.31 cm x 2.3 cm =
3.0 cm2
The annual production of sodium hydroxide (NaOH) in the United States in 2019 was 54.6 billion liters. How pounds of sodium hydroxide are produced? (density of sodium hydroxide is 2.130 g/cm3).
If the same mass of barium hydroxide is needed, what volume (in m3) would need to be produced. (density barium hydroxide is 3.74 g/cm3)
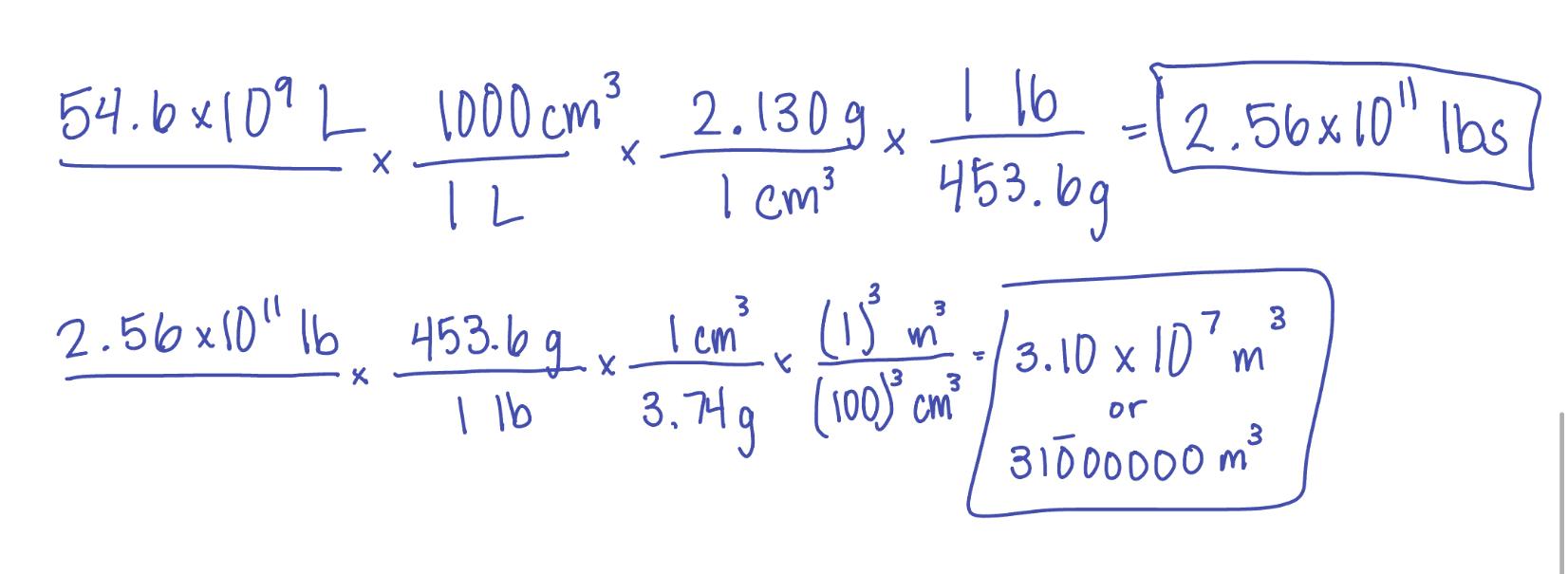
The percent error of a student who calculated the density of water as 0.98 g/mL when the actual density of water is 1.00 g/mL.
What is -2.0% error? (please ignore the absolute value in my work)