Matter requires
What is mass and volume?
The EPOCH acronym can be used to determine if a reaction is this.
What is chemical?
Chromatography can be used to separate this kind of mixture.
What is homogeneous?
During this transition a substances changes from a solid to a liquid.
What is melting?
Volume is an example of this type of property.
What is an extrinsic physical property?
The flat parts of a heating/cooling curve indicate this.
What is a phase transition?
Heat and light
What is energy?
The sublimation of dry ice is this kind of reaction.
What is physical?
What is a solution?
During this transition, particles in a liquid gain kinetic energy.
What is vaporization?
Color, density, hardness.
What are examples of intrinsic physical properties?
The phase of the substance will be indicated in this part of the heating/cooling curve.
What is the sloped part of the line?
Atoms of an element could be considered this.
What is a pure substance?
Chewing your food is this kind of reaction.
What is physical.
A suspension could be separated without using heat by this techique.
What is filtration?
The particles in this phase of matter has the least amount of kinetic energy.
What is a solid?
This type of property will exhibit one or more of the EPOCH changes.
What is a chemical property?
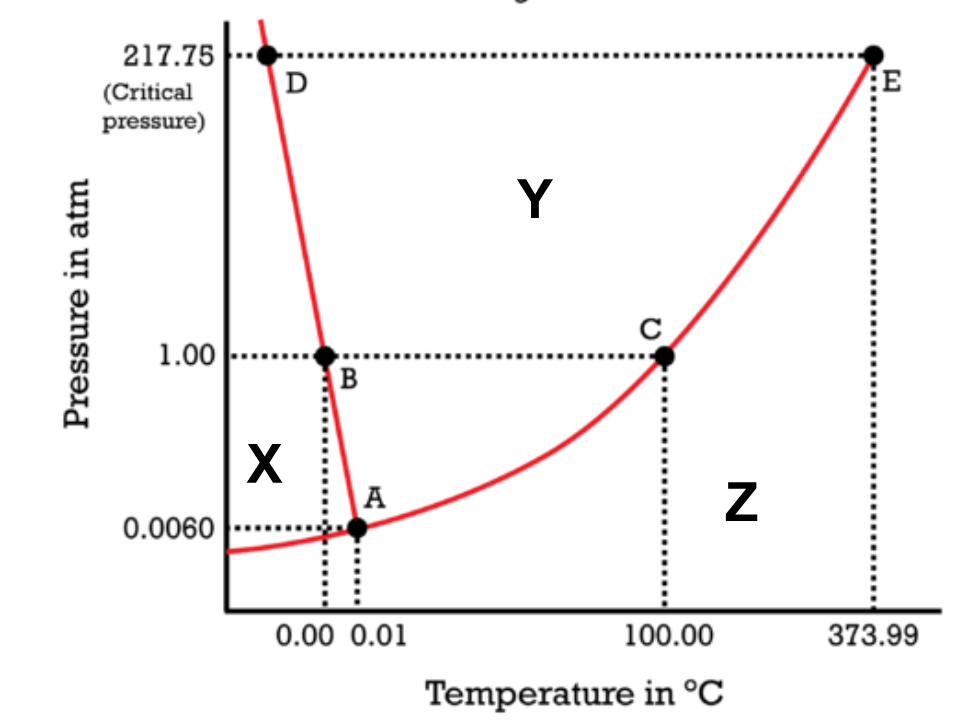
The phase of matter represented by the letter "Y".
What is liquid?
Distilled water could be considered this.
What is a pure compound?
What is physical?
This transition converts a solid directly to a gas.
What is sublimation?
A fancy word for bubbles.
What is effervescence?

The phase of matter at 0.0060 atm and 100 degrees Celcius.
What is gas?
A homogeneous mixture that will not separate and exhibits the Tyndal effect.
What is a colloid?
A the chemical reaction between two clear liquids produces a white chalky substance in the beaker. The white substance is this.
What is a precipitate?
Water and grease do not want to mix. Soap acts as this so the water and grease can mix and you can clean your hands.
What is an emulsifying agent?
The three phase transitions that require energy to be added to the system.
What are: melting, vaporization and sublimation?
Bromothymol blue changing to yellow when an acid present is an example of this type of property.
What is a chemical property?

Boiling point will do this if you decrease the pressure.
What is decrease?