In this state of matter, molecules are tightly packed in a regular pattern. They vibrate but they can not move past each other.
What is a SOLID
If the molecules of a sample speed up, what else happens?
The temperature of the sample increases.
The kinetic energy of the molecules increases.
When the temperature of matter increases what happens to the molecules?
They speed up and move farther apart.
Which model best represents a solid at a higher temperature.
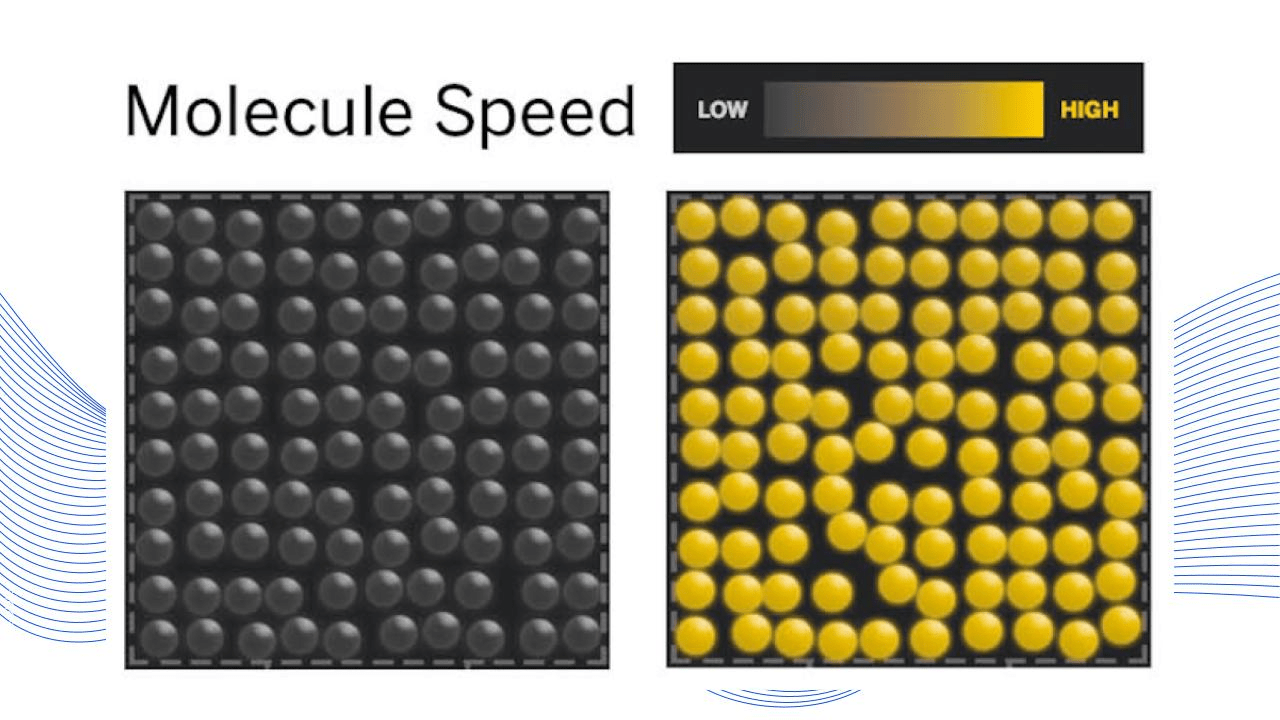
What is the right model
The right model has molecules that are yellow. Yellow indicates molecules are moving at a high speed, therefore the average kinetic energy (temperature) is higher.
What can you infer will happen to the molecules of a substance that is cooled.
The molecules will slow down.
The molecules will lose kinetic energy.
MISCONCEPTION: They will stop moving. Molecules only stop moving at absolute zero.
In this state of matter, molecules take the shape of its container, have no regular pattern, and move freely at high speeds
What is a GAS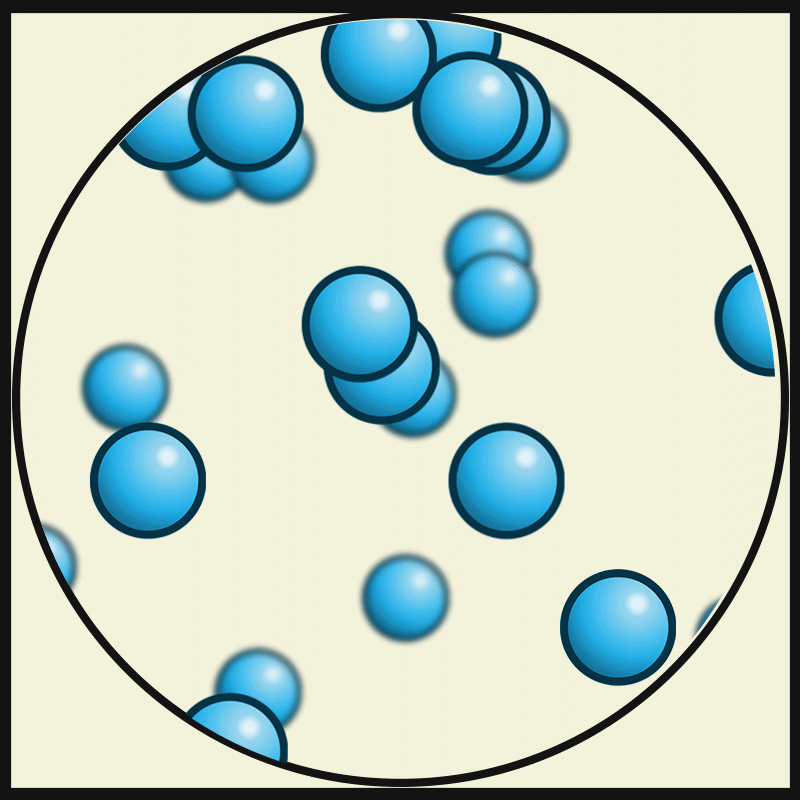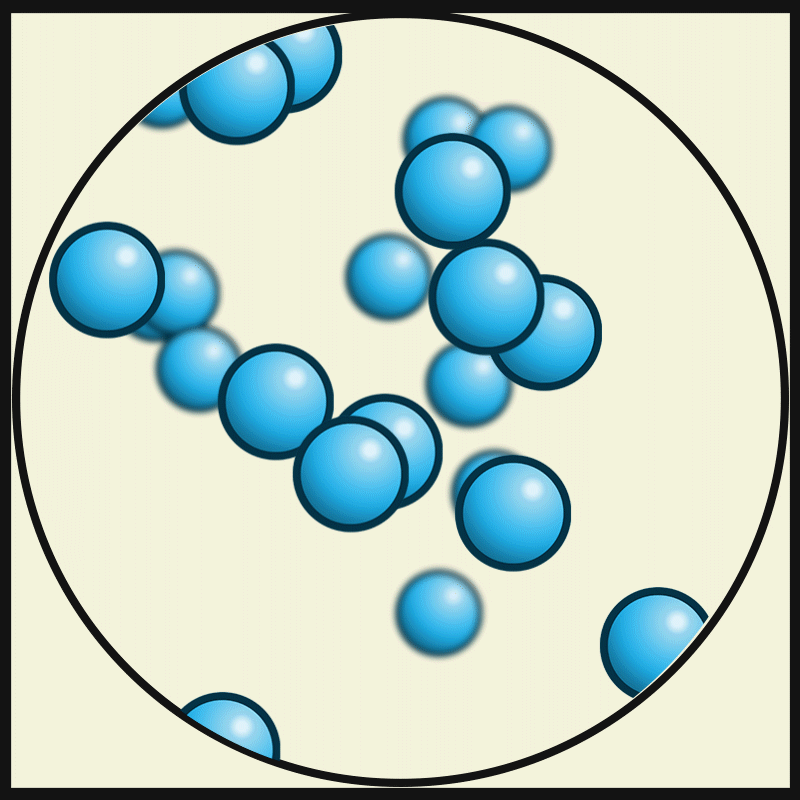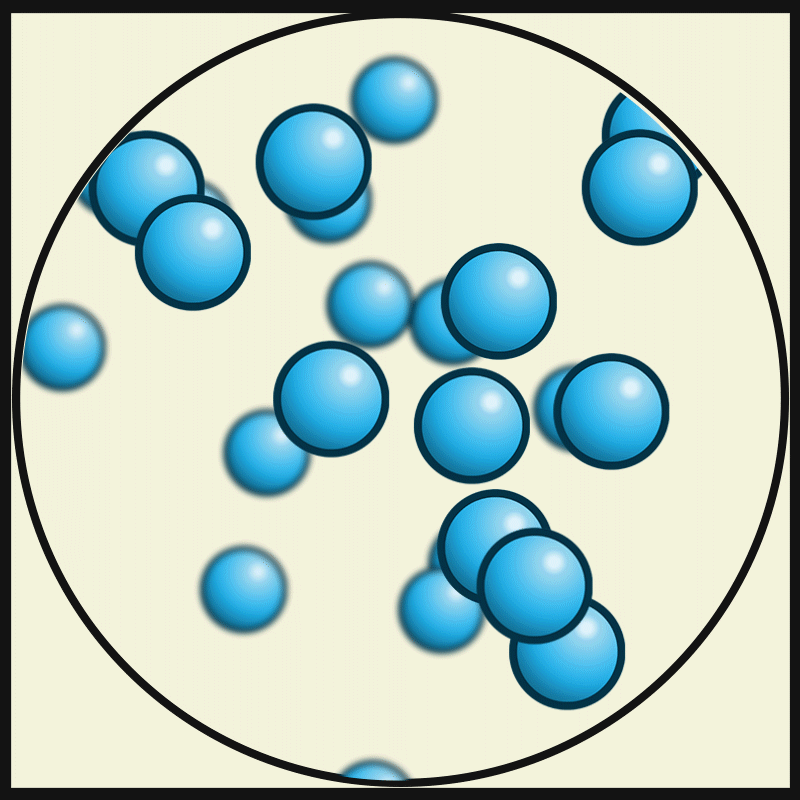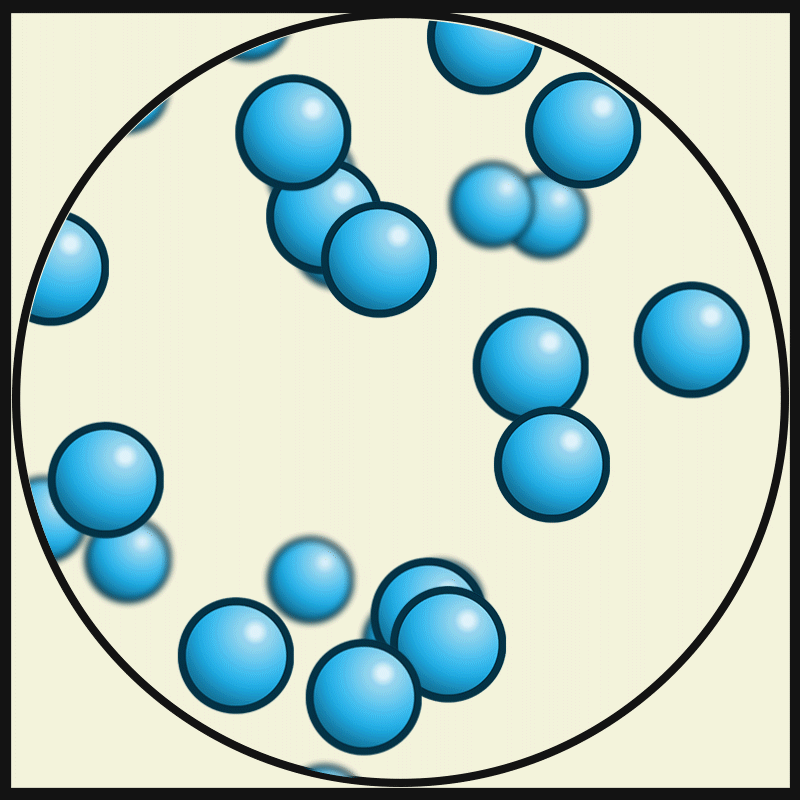
The TOTAL kinetic energy of the molecules of a thing
What is thermal energy
The process that changes a liquid to a gas
What is evaporation

Milo puts a marshmallow into his hot chocolate. After a while the temperature of the marshmallow increases. What happens to the molecules of the marshmallow when the temperature of the marshmallow increases?
What is...
the kinetic energy of the molecules increases
the molecules speed up
State(s) of matter that has molecules that have no regular pattern and take the shape of the container.
What is a liquid and a gas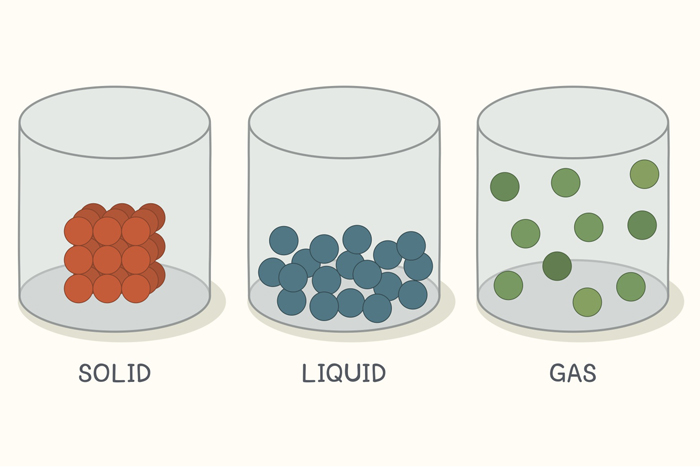
When two things are in contact, kinetic energy transfers...
What is from fast-moving molecules to slower-moving molecules.
Freezing occurs when you ___ thermal energy
What is remove, decrease
The object with a higher temperature.
What is A because the molecules have the highest average kinetic energy.
The molecules in A are moving faster on average than sample B and C.
 After taking a hot shower, James steps out of the shower onto the tile floor. The tile felt cold to his feet. What can you infer about James' experience.
After taking a hot shower, James steps out of the shower onto the tile floor. The tile felt cold to his feet. What can you infer about James' experience.Energy transferred from his feet to the tile floor.
How is the arrangement of water molecules different than other substances when it is solid (ice)?
Water expands when it is solid.
Water molecules move slightly further apart when it freezes as they arrange themselves in fixed positions.
HOW does energy transfer from one object to another?
When molecules collide energy transfer from faster moving molecules to slower moving molecules.
This phase change occurs when the molecules of a sample speed up enough that the motion overcomes the attractions so that the molecules can move past each other
What is melting
The state of matter represented by the model
What is plasma
What can you infer will happen when the top box and bottom box touch?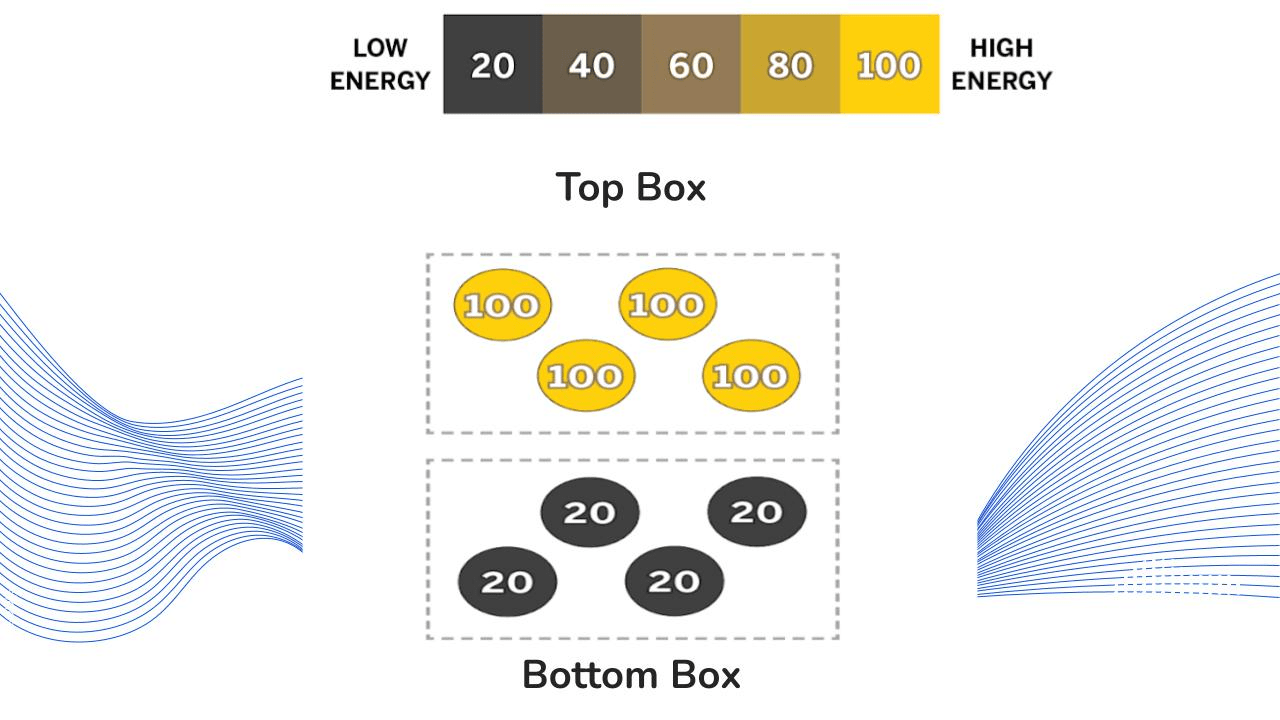
The top box will transfer kinetic energy to the bottom box.
The bottom box will become hotter and the top box will become colder.
Kinetic energy will transfer until they reach the same temperature (equilibrium).
The molecules in the top box will collide with the molecules in the bottom box.
This state of matter occurs ONLY at Absolute Zero, when molecules lose all their energy and stop moving.
What is Bose Einstein Condensate
The point at which the molecules of a system stop transferring energy
What is equilibrium, when all the molecules are moving at the same speed
What phase change does the model represent?

What is Condensation
Which systems will change if the samples come into contact?
What is system 1 and system 4
In both system 1 and system 4 there are two samples at different temperatures. When the two samples come into contact faster moving molecules will collide and transfer kinetic energy to the slower moving molecules until they reach equilibrium.
If you add an ice cube to 1) a large bowl of soup, and 2) a smaller mug of soup, what will the impact be?
Vocab: temperature, decrease, increase, equilibrium, thermal energy, transfer
Both soups will decrease in temperature, and the ice will increase in temperature. The ice cube in the bowl of soup will reach a higher equilibrium temperature because the bowl of soup has thermal energy to transfer to the ice cube.
