Draw Dalton's model of the atom.

79 protons
Does nickel-58 or nickel-64 have a greater atomic mass?
What is the average atomic mass of arsenic?
74.922 amu
What were the two key discoveries from J.J. Thomson's cathode ray experiment?
1. There are particles that are smaller than the atom.
2. There are negative electrons in the atom.
What is the atomic number of zirconium?
40
How many protons, electrons, and neutrons are in the following isotope? What is the mass number of the isotope?
P: 36 protons
E: 36 electrons
N: 42 neutrons
Mass #: 78
Which element has an atomic mass of 131.29 amu?
Xenon
Draw Rutherford's atomic model. Include labels in your drawing!
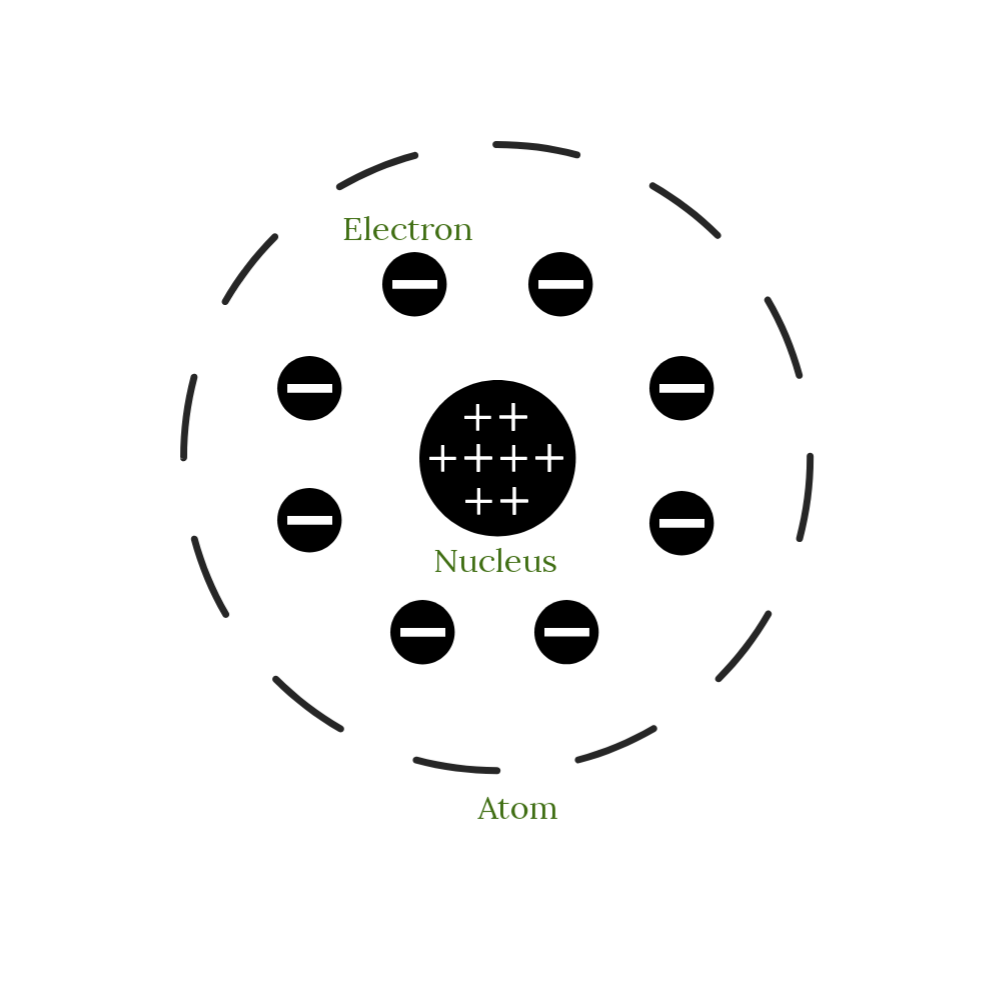
Which element contains 84 protons?
Polonium
How many protons, neutrons, and electrons are in a neutral atom of vanadium-50?
P: 23 protons
N: 27 neutrons
E: 23 electrons
Calculate the average atomic mass of lithium, which occurs as two isotopes. Lithium-6 has an abundance of 7.30% and a mass of 6.017 amu, and lithium-7 has an abundance of 92.70% and a mass of 7.018 amu.
6.94 amu
Draw Thomson's atomic model. Include labels in your drawing!

What is atomic number equal to?
Number of protons in the atom.
Write the isotope notation for the following atom. 
1123Na
The element copper has naturally occurring isotopes. The relative abundance and atomic mass are 69.2% for a mass of 62.93 amu and 30.8% for a mass of 64.93 amu. Calculate the average atomic mass of copper.
63.546 amu
What were the two key discoveries from Ernest Rutherford's gold foil experiment?
1. The atom is composed of mostly empty space.
2. The atom contains a positive nucleus.
How many electrons does a neutral atom of francium have?
87 electrons
Write the isotope notation for copper-63.

Calculate the average atomic mass of iridium using the following data for two iridium isotopes. Iridium-191 has an abundance of 37.58% and mass of 191.0 amu. Iridium-193 has an abundance of 62.42% and a mass of 193.0 amu.
192.25 amu