Is Mercury an element, compound, or mixture?
Element
How is a gas defined?
Indefinite shape, indefinite volume
This type of graph is called a:
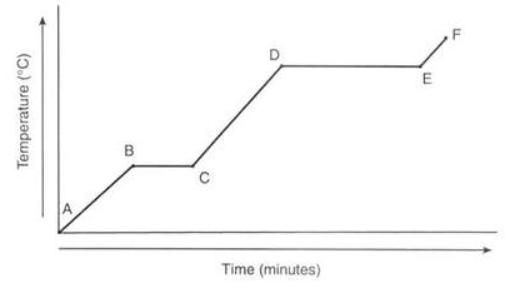
Heating Curve
Water can boil at 100 degrees celsius. What type of change is this?
Physical Change
What is density?
Density is the mass of an object divided by its volume
Is table salt, sodium chloride, an element, a compound or a mixture?
Compound
_____________________ takes place when a liquid changes to a solid.
Freezing
Between which points is the temperature of the substance remaining constant? 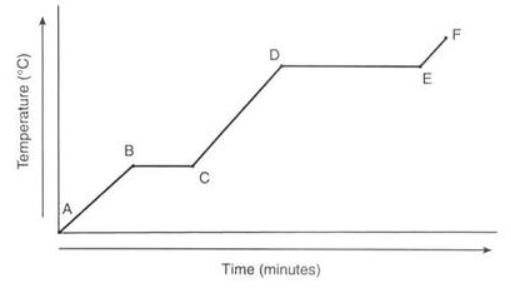
B-C and D-E
Iron rusts, producing a layer of oxide on the outside of the metal.
Chemical Change
What is viscosity?
A liquid's resistance to flow.
What type of a mixture is Coffee?
Homogenous Mixture
What term describes a solid changing to a gas?
What line segment represents only the solid-state?
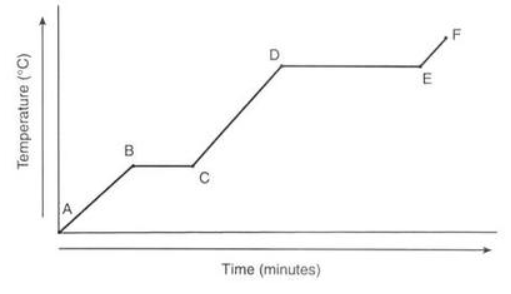
A-B
When sugar dissolves into water...
Physical Change
What units are used to measure Density?
g/cm3
A type of matter with a fixed composition?
Pure Substance
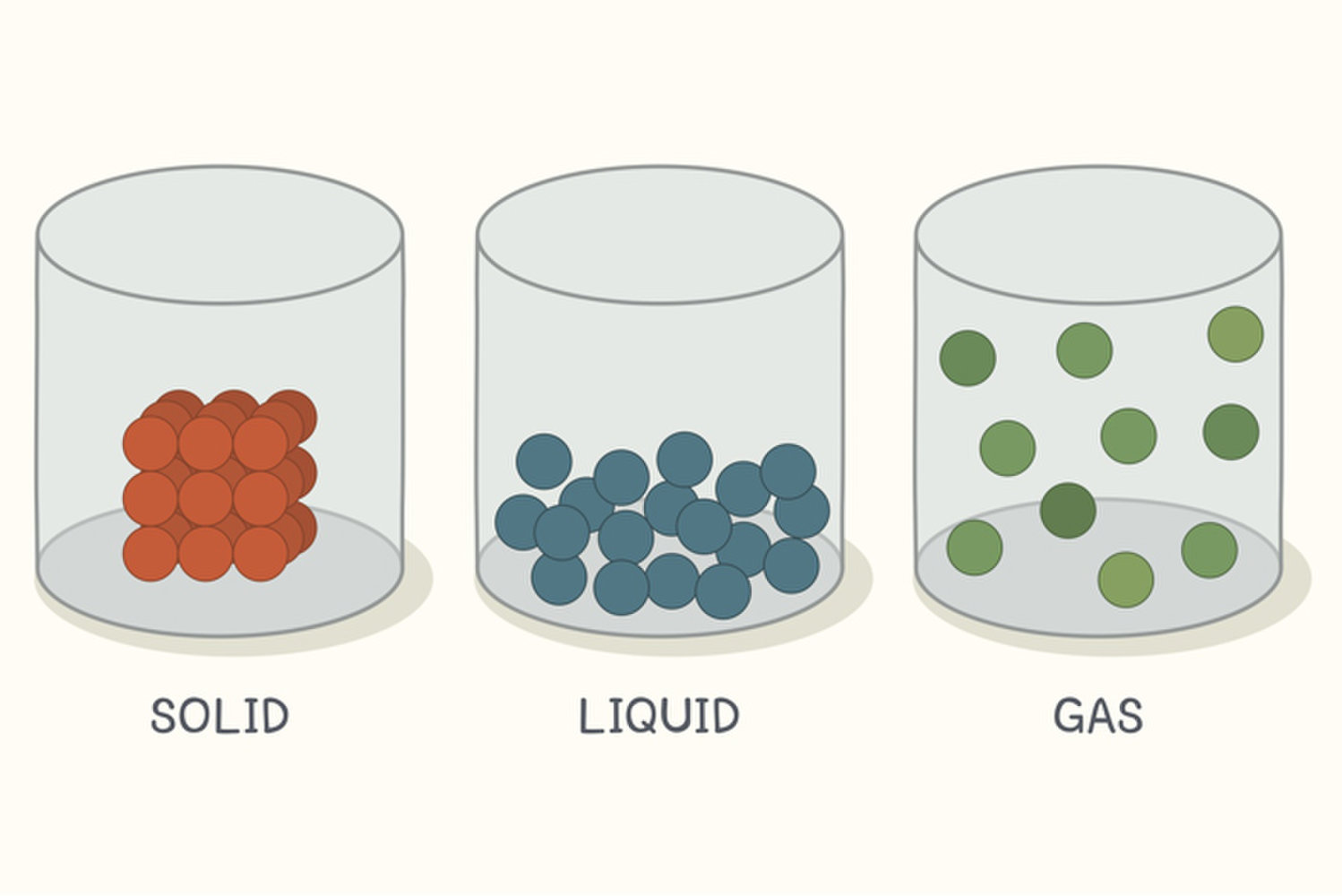
Draw particles of a solid, liquid, and gas.
What state(s) of matter are present at D-E?
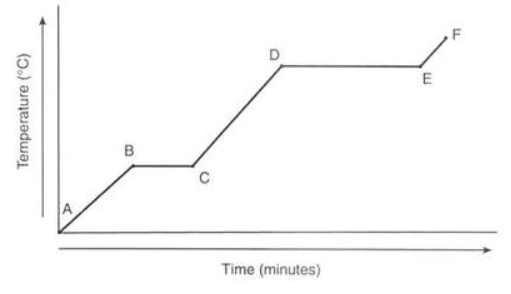
Liquid-Gas
Vinegar reacts with baking soda to create a brand new gas (Carbon Dioxide)
Chemical Change
True or False?
Honey has a greater viscosity than vinegar.
True
Matter is defined as anything that _____
has mass and takes up space.
What happens when my glass of water is sitting in the sun and disappears into a gas?
Evaporation
Which process takes the longest to occur?
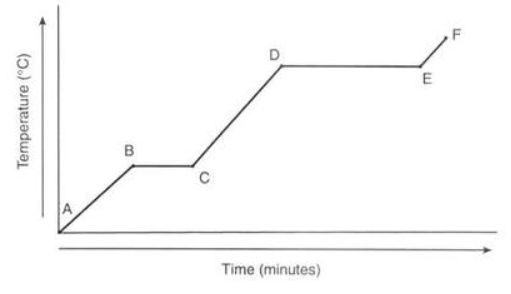
Boiling
What happens in ALL chemical changes?
A new substance is formed.
If 2 objects with the same mass are placed into water tanks...
-object A displaces 10mL of water
-object B displaces 15mL of water
Which object is denser?
Object B