What is the law of conservation of mass?
Matter cannot be created or destroyed.
How can you determine the density from a mass vs volume graph?
Density is the slope of the line
Which box has the largest volume?
C
A metal has a volume of 15cm3 and density of 3.0g/cm3. What is it's mass?
45 g
What is the volume of an object that made the water level rise in a graduated cylinder from 50 mL to 59 mL?
9 mL
When we burned steel wool, the mass increased. Did we create mass? Explain
No! The increase in mass was due to air particles reacting with the steel wool.
When comparing two material's lines of best fit on a mass vs volume graph, how do you know which material is denser?
The denser material has a steeper slope
Which box has the highest mass of green particles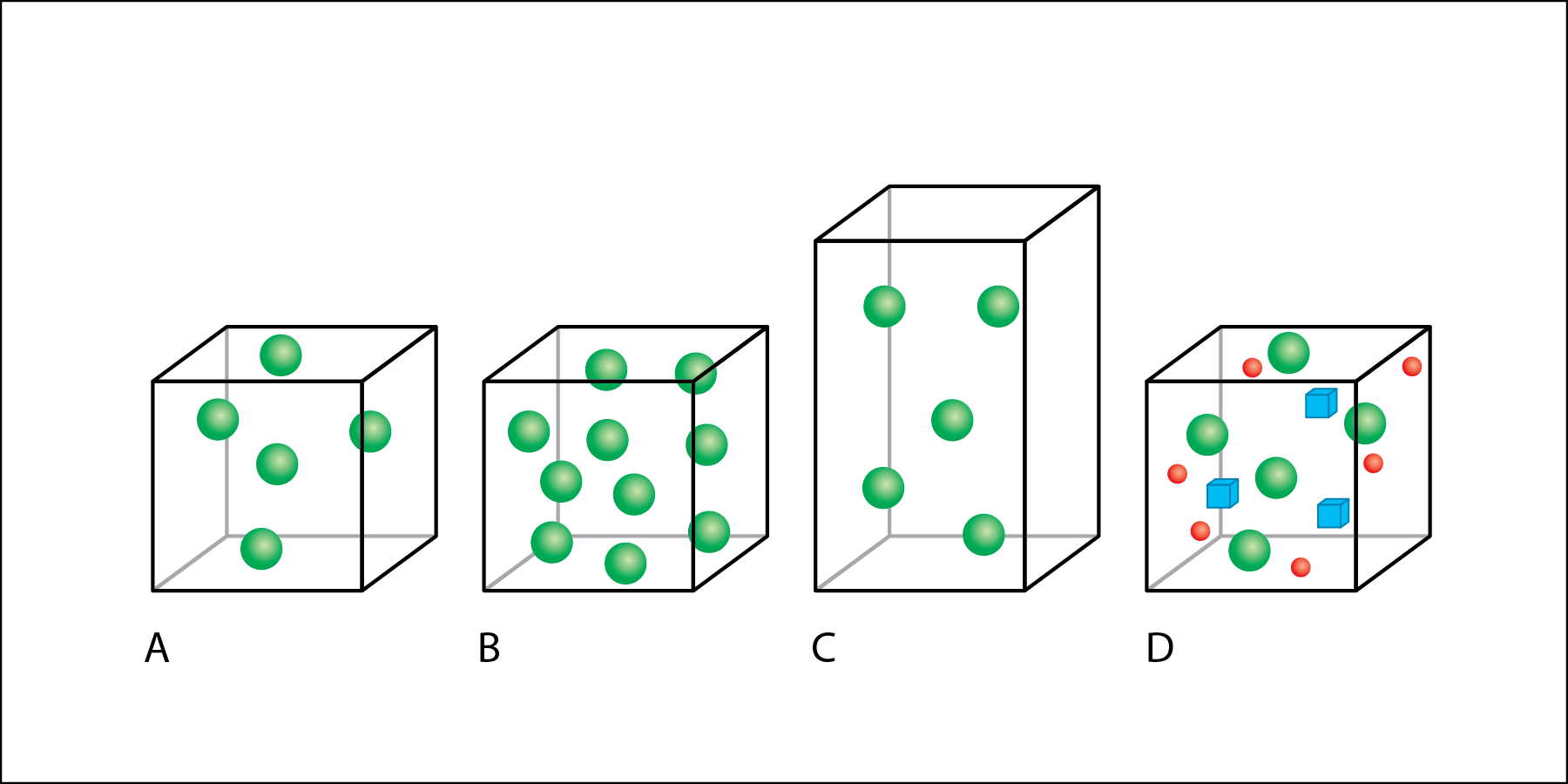 ?
?
B
A liquid has a mass of 24g and density of 2.0g/mL. What is it's volume?
12 mL
If there are two liquids, A and B, in a beaker and B is floating on top of A, what is true about their density?
B is less dense than A
You leave a cup of water outside for a couple of days. When you come back, you find that there is less water in the cup than before. Was matter destroyed?
No, some water evaporated from the cup.
Which material is denser?
A
Which box has the lowest density of green particles?

C
A cube of gold-colored metal with a volume of 54 cm3 has a mass of 980 g. The density of gold is 19.3 g/cm3. Is this sample of metal pure gold? Why or why not?
No, the density of this metal is 18.1 g/cm3, not 19.3g/cm3
How does the density of a gas compare to the density of solids and liquids?
Gases are 1000 times less dense than solids and liquids
How can the Law of Conservation of Mass apply to a large burning log, if all that remains of it is a small pile of ash at the end?
The matter from the log became smoke (and some ash remained)
According to the graph, if you have 3 cm3 of copper, what’s the mass?

~25 g
Wood has a density of 0.85 g/mL and lead has a density of 11.3 g/mL. If I have a block of wood and a block of lead that have the same volume, which one will have a larger mass?
Lead
The density of silver is 10.5 g/mL. What’s the volume of 2500 g of silver?
238 mL
Will different volumes of water always have the same density? Why or why not?
Yes, density doesn't change no matter how much of it you have
carbon dioxide!
What is the density of copper according to the graph?
~9 g/cm3
Wood has a density of 0.85 g/mL and lead has a density of 11.3 g/mL. If I have a block of wood and lead that have the same mass, which one will have the larger volume?
Wood
You decide you want to carry a boulder home from the beach. It has a volume of 27,000 cm3. It is made of granite, which has a typical density of 2.8 g/cm3. How much will this boulder weigh?
75,600 g
Will an object with a density of 0.86 g/cm3 sink or float in water? How do you know?
Float, the density of water is 1 g/mL, so it is less dense than water.