What is mass?
The amount of matter something has.
What is volume?
The amount of space something takes up.
What is the equation for density?
D=m/v
Fernando measures out 25 mL of water. How many liters is this? (1000 mL = 1 L)
0.025 L
Convert 450,000 mm to km.
0.45 km
What tool do we use to determine the mass of something?
Balance
What lab equipment is most often used to determine the volume of a liquid?
Graduated Cylinder
You find an object while hiking in the woods. It has a mass of 25g. You placed it into a graduated cylinder with 10 mL of water and the volume changed to 20 mL. What is its density?
2.5 g/mL
A stone weighs 453 kg. How many grams is this? (1000g = 1 kg)
453,000 g
Convert 52 km to mm.
52,000,000 mm
How would you find the mass of this calcium carbonate?

Place weighboat on balance.
Hit tare/zero to clear the weight of the boat.
Place to calcium carbonate in the boat and place it back on the balance.
What is the volume of the water in this graduated cylinder?

15 mL
Mercury is liquid at room temperature. It has a density of 13.6 g/mL. You have 10 mL of mercury. What is its mass?
136 g
How many meters are in 500 cm? (100 cm = 1 m)
5 m
How many hours are in 1 week?
168 hr
How would we take the mass of this block?

Place it on a balance and read the output.
What is the volume of the tiny hammer?
4 mL
Tin has a density of 7.31 g/mL. You have a sample of tin that has a mass of 2.3 g. What is its volume?
0.31 mL
How many liters are in 1,532 mL of mercury? (1000 mL = 1 L)
1.532 L
How many inches are in 2 miles?
1 mi = 5280 ft
12 in = 1 ft
126,720 in
How would you take the mass of these orbeez?

Place weighboat on balance.
Hit tare/zero to clear the weight of the boat.
Place to orbeez in the boat and place it back on the balance.
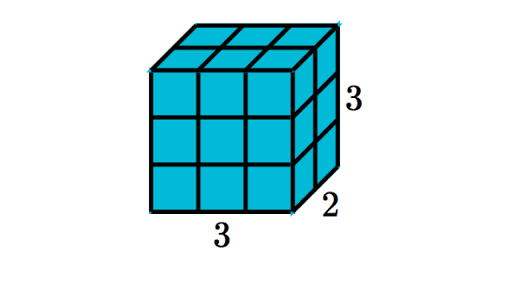
What is the volume of this block? Units are in cm.
18 cm3
Platinum has a density of 21.45 g/cm3. You find a ring while laying on the beach. You determine it has a mass of 52.0 g and a volume of 2 mL. Is the ring made of platinum?
No, the density of the ring is 26 g/mL, while platinum has a density of 21.45 g/mL.
How many mg are in 5.23 g of a substance? (1000 mg = 1 g)
5,230 mg
How many days are in 500,000 seconds?
5.79 days