What did science first start out as?
Philosophy
What is the charge on a:
Proton?
Neutron?
Electron?
Proton: +
Neutron: Neutral/no charge
Electron: -
How many protons and electrons are in: 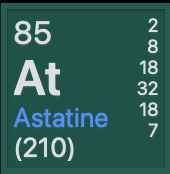
85
What is included in standard notation?
The element name and the mass number
What is the mass of this element?
51.996 or 52
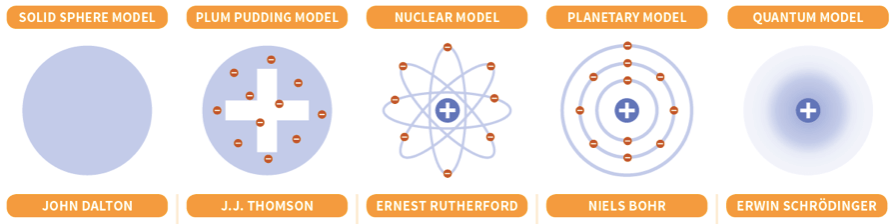
What were the names of the scientists who contributed to atomic theory?
Dalton, Thomson, Rutherford, Bohr, and Schrodinger
Where are protons, neutrons, and electrons located?
Protons & neutrons are located in the nucleus. Electrons are located in energy levels in the space outside the nucleus.
What is difference between the valence electrons in groups 3-12 compared to groups 1,2,13-18?
Groups 3-12 have ranges of valence electrons whereas groups 1,2,13-18 have a fixed amount.
What do protons and electrons tell you?
Protons tell you the element
What is the smallest subatomic particle, does it contribute to the mass number?
An electron, no it does not
Who performed the Gold Foil Experiment?
Rutherford
What are valence electrons?
An electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed
How do you determine the number of neutrons in an atom?
Mass # - Atomic # = # of Neutrons
What does a Lewis Dot Diagram tell us?
How many valence electrons an atom of an element has.
What does the atomic mass consist of?
The number of protons and the number of neutrons
What was Bohr's contribution to atomic theory?
Bohr proposed that the electrons were only found in specific circular paths or orbits around the nucleus in energy levels.
What does it mean when an electron is in its ground state?
The electron is at its lowest possible energy level
What is tungstens atomic number, mass # , and symbol?

What is an isotope?
Isotopes are atoms that have the same number of protons and different numbers of neutrons.
Why is the atomic mass a weighted average?
The atomic mass of an element is a weighted average mass of the atoms in a naturally occurring sample of the element. The weighted average reflects both the mass and the relative abundance of the isotopes as they occur in nature.
Which scientist created each atomic model? 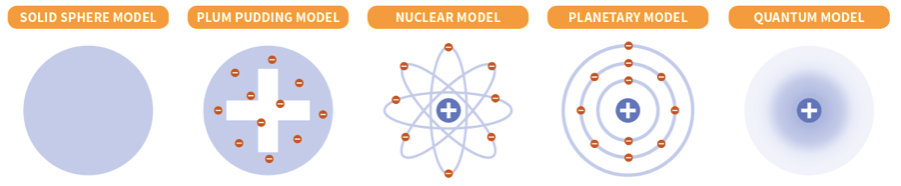
What is emission spectra?
The spectrum of light released by electrons of atoms of a particular element when they fall back to their ground state energy level after being excited.
How many neutrons and valence electrons are in an atom of Gallium?
Mass # - Atomic # = # of neutrons
70 - 31 = 39 neutrons
Gallium is part of group 13
Gallium has 3 valence electrons
Write the following element in standard notation, nuclear notation, and draw its Lewis Dot Diagram.
Polonium-209
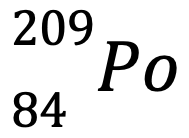

Hydrogen is 99% Hydrogen-1, 0.8% Hydrogen-2, and 0.2% Hydrogen-3. Calculate its average atomic mass.
Hydrogen-1
- 1 x .99 = .99
Hydrogen-2 Avg. Atomic Mass
- 2 x .008 = .016 .99 + .016 + .006 = 1.012
Hydrogen-3
- 3 x .002 = .006