Which of the 3 subatomic particles has a positive charge?
Protons
What is the name of the small dense structure is in the center of the atom that holds the protons and the neutrons?
Nucleus
What is represented by the letter A?
Atomic Number
What determines the identity of an atom?
(what do I need in order to go find it on the periodic table?)
The number of protons which is the Atomic Number
Valence Electrons
The up and down columns are called ________
Groups
I am an electrically neutral atom that has an atomic number of 5. Who am I?
Boron (B)
Which of the 3 subatomic particles has a neutral charge (no charge)
Neutrons
What is the name of the structure of an atom that is outside the nucleus and contains the electrons?
Electron cloud
What does the atomic number directly refer to?
Number of protons
What is the atomic number of this atom?
3
Because there are 3 protons
If an atom has an outer shell that is full of valence electrons (8) would it be considered highly reactive or non-reactive?
Non-reactive
Full shell is non-reactive and stable
The side to side rows are called _______ and tell you how many ________ ________ an element has
Periods
Energy levels (rings)
could also be called shells
I am an electrically neutral atom that contains 9 protons, 9 electrons and 10 neutrons. Who am I?
Fluorine (F)
Which of the 3 subatomic particles has a negative charge
Electrons
An atom is made up of mostly _______ _______
Empty Space
What else does the atomic number tell you?
The number of electrons
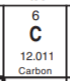
What is the identity of this atom?
Carbon (C)
Because there are 6 protons in the model and Carbon has atomic number 6
If an atom has only 1 valence electron in its outer shell, would it be considered highly reactive or non-reactive?
Highly reactive
The less valence electrons the more reactive (angry)
What color is the box that surrounds the Group number?
Blue
Group number is the top and has no letters
I am an electrically neutral atom that contains 4 neutrons, 3 electrons, and some protons. Who am I?
Lithium (Li)
Which 2 subatomic particles are located inside the nucleus?
Protons and Neutrons
Which structure makes up most of an atoms mass?
Nucleus
Because the only particles that have mass (protons and neutrons) are in the nucleus
What determines the identity of an atom?
(what do I need in order to go find it on the periodic table?)
The number of protons which is the Atomic Number
What is the identity of this atom?
Helium (He)
Because it has 2 protons which means it has an atomic number of 2

Where are valence electrons located?
Outer most energy level
What color is the box that surrounds the period number?
Red
Period number tells you how many energy levels (shells or rings) every element has in that row (all the way across)
What is my identity (who am I)
Beryllium (Be)
Which subatomic particle orbits around the nucleus on the energy levels in the electron cloud?
Electrons
Which structure makes up most of an atoms volume?
Electron cloud
Electron cloud is full of empty space and is what makes the atom take up space
How many protons does this element contain?
How many electrons does this element contain?

11 protons and 11 electrons
Because the atomic number is 11 and atomic number tells you how many protons and also how many electrons
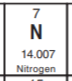
What is the identity of this atom?
Nitrogen (N)
Because it has 7 protons which means it has an atomic number of 7
How many valence electrons does this atom contain?
3
Count the electrons on the outermost shell
They have the same number of energy levels.
They both have 3 energy levels
What is my identity (who am I)
Neon (Ne)
Which 2 subatomic particles both have a mass of 1amu?
Protons and Neutrons
If the nucleus is full of protons with a positive charge, and neutrons with a neutral (no charge) charge. What is the charge of the entire nucleus?
Positive
Positive charges and no charges = positive charge
How many protons does this element have?
How many electrons does this element have?
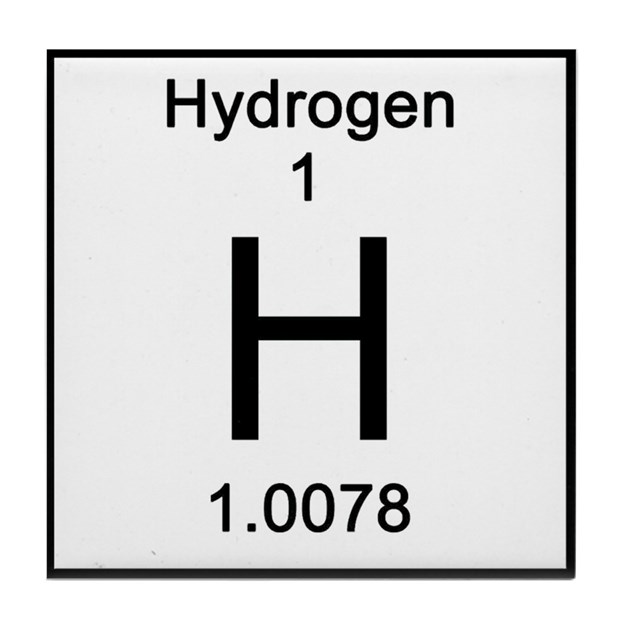
1 proton and 1 electron
Because the atomic number is 1 and atomic number tells you how many protons and also how many electrons
An electrically neutral atom contains 5 protons, 5 electrons, and 6 neutrons. What is the identity of this atom?
Boron (B)
5 protons means it has an atomic number of 5

Which colored box tells you how many valence electrons that column of elements contains
Green box
The numbers that have the letter "A" next to them tell you how many valence electrons
If two elements are in the same group what do they have in common?
They have the same number of _________
Same number of valence electrons
I am in group 17.
I have 7 valence electrons and 3 energy levels
Who am I?
Chlorine (Cl)
Which subatomic particle has a mass of 0amu?
Electrons
If the electron cloud is full of empty space and electrons, what is the charge of entire electron cloud?
Negative
Because electrons have a negative charge
What is represented by letter B?
Atomic Mass
Identify the element that has 10 protons
Neon (Ne)
10 protons means it has atomic number 10

Which element is more reactive, Potassium (K) or Krypton (Kr)?
Potassium (K) is more reactive
When you find potassium go to the top of the column and find the number with the letter "A".
Potassium (K) is under 1A meaning it has 1 valence which makes it highly reactive
Krypton (Kr) is under 8A which means it has 8 valence electrons. 8 is a full shell which makes it non reactive
3 valence electrons
The valence number under the group number says 3A which means they all have 3 valence electrons
I am in Group 13
I have 3 valence electrons and 4 energy levels
Who am I?
Gallium (Ga)
Which of the 3 subatomic particles has the smallest mass?
Electrons
Protons and electrons
same number of positive particles (protons) and negative particles (electrons)
What does the atomic mass tell you?
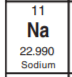
Identify the that has 11 protons and 12 neutrons
Sodium (Na)
11 protons means it has an atomic number of 11
Which family on the periodic table is the most reactive?
Alkali Metals (Group 1)
Because they are all under 1A meaning they all have 1 valence electron and are highly reactive.
What is the Family name of Group 1?
Alkali Metals
Most reactive family
What is my identity? (who am I)
Argon (Ar)
8 valence electrons and 3 energy levels
Where are valence electrons located?
On the outermost energy level (ring)
Is this atom electrically neutral?
Yes
3 protons (positive) and 3 electrons (negative)
How man protons does this element have?
How many electrons does this element have?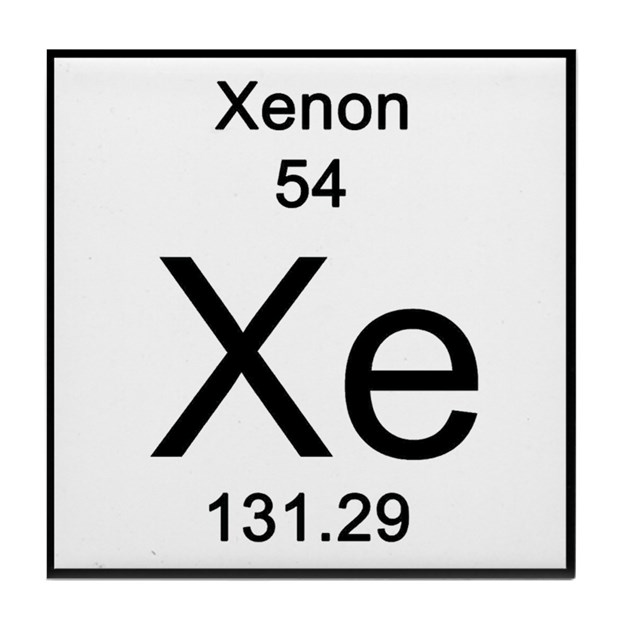
54 protons
54 electrons
Atomic number directly refers to number of protons
Atomic number also tells you how many electrons
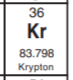
Identify the element that has 36 protons
Krypton (Kr)
36 protons means it has an atomic number of 36
Which family on the periodic table is the least reactive?
Noble Gases
Because they are under 8A which means they all have 8 valence electrons (besides He) and full outer shells.
What is the Family name of Group 2?
Alkaline Earth Metals
What is my identity? (Who am I)
Boron (B)
2 energy levels and 3 valence electrons
If the nucleus is full of protons with a positive charge, and neutrons with a neutral (no charge) charge. What is the charge of the entire nucleus?
Positive
Positive charges and no charges = positive charge
How do you find the mass of an atom?
Add everything in the nucleus together (protons + neutrons)
How many protons does this element have?
How many electrons does this element have?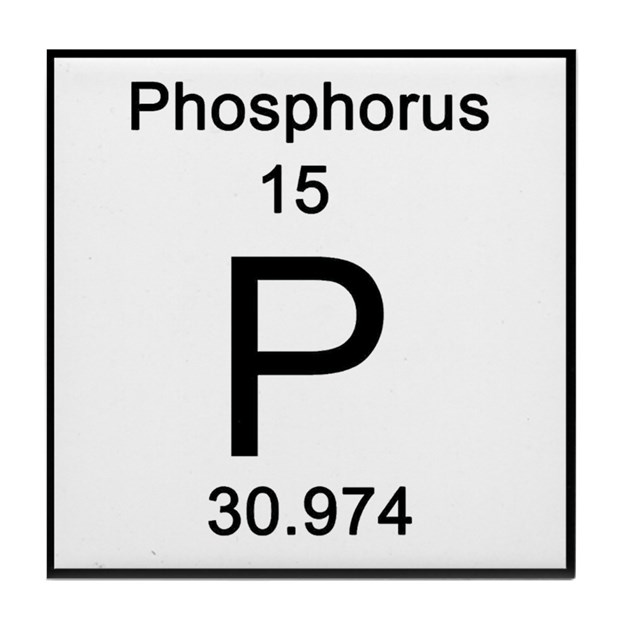
15 protons
15 electrons
Atomic number directly refers to the number of protons
Atomic number also tells you how many electrons
Identify the element that has 33 protons and 42 neutrons.
Arsenic (Ar)
Why is Argon (Ar) less likely to react than Sodium (Na)?
Because Argon (Ar) has a full outer shell
What is the Family name of Group 17?
Halogens
I am in the Noble gases family and I have 5 energy levels
Who am I?
Xenon (Xe)
Group 18, period 5
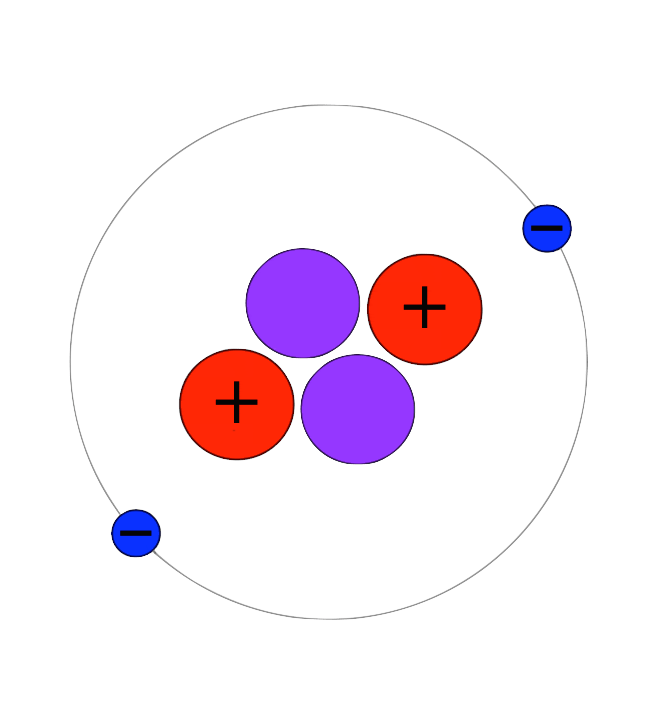
Can you identify the subatomic particles represented by letter A, B, C?
A = Electron (outside the nucleus)
B = Neutron (in the nucleus but no charge)
C = Proton (in the nucleus with a positive charge)
What is the mass of this atom?
7
3 protons + 4 neutrons = mass of 7
How many neutrons does this element have?

6
Round the atomic mass to 11.
Atomic mass - atomic number = neutrons
11-5=6
What is the identity of an electrically neutral atom that contains 26 neutrons, 22 electrons and some protons?
Titanium (Ti)
You didn't have protons, but in an electrically neutral atom the protons and electrons are the same number
Describe the reactivity of this element (highly reactive, moderately reactive, or non-reactive)
Moderately reactive
What is the Family name of Group 18?
Noble Gases
I am in the Alkaline Earth Metals family and I have 3 energy levels
Who am I?
Magnesium (Mg)