Atoms have the same amount of
Protons and Electrons
What type of bond does magnesium and chlorine have?
What are the oxidation numbers after they bond?
Ionic
Mg+2 and Cl-1
Ionic bonds are between
a metal and non metal
Covalent bonds are between
A non metal and non metal
Draw the Lewis dot model for Cesium (Cs)
C .
Metals are on the _____ side of the periodic table
Left
SiO2
Ms. Hali's favorite compound, has what type of bond? Oh and what kind of melting point does it have?
SiO2 is a covalent bond and has a low melting point.
Which one of these pairs are ionic?
Argon and Oxygen
Iodine and Boron
Polonium and Oxygen
Copper and Sulfur
Copper and Sulfur
(metal and non metal)
Can take the physical form of
Gas/Liquid/Solid
What is the Oxidation number for Neon?
0
Metalloids have the same physical properties as _____ and same chemical properties as _____?
Metals, Nonmetals
Single, Double, and Triple bonds are
Covalent
Ionic compounds always form as
Crystals
How do you represents bonded pairs of electrons?
With a dash
1 dash- 2 shared electrons (single)
2 dash- 4 shared electrons (double)
3 dash- 6 shared electrons (triple)
Draw the Lewis dot bonds for Magnesium and Chlorine.
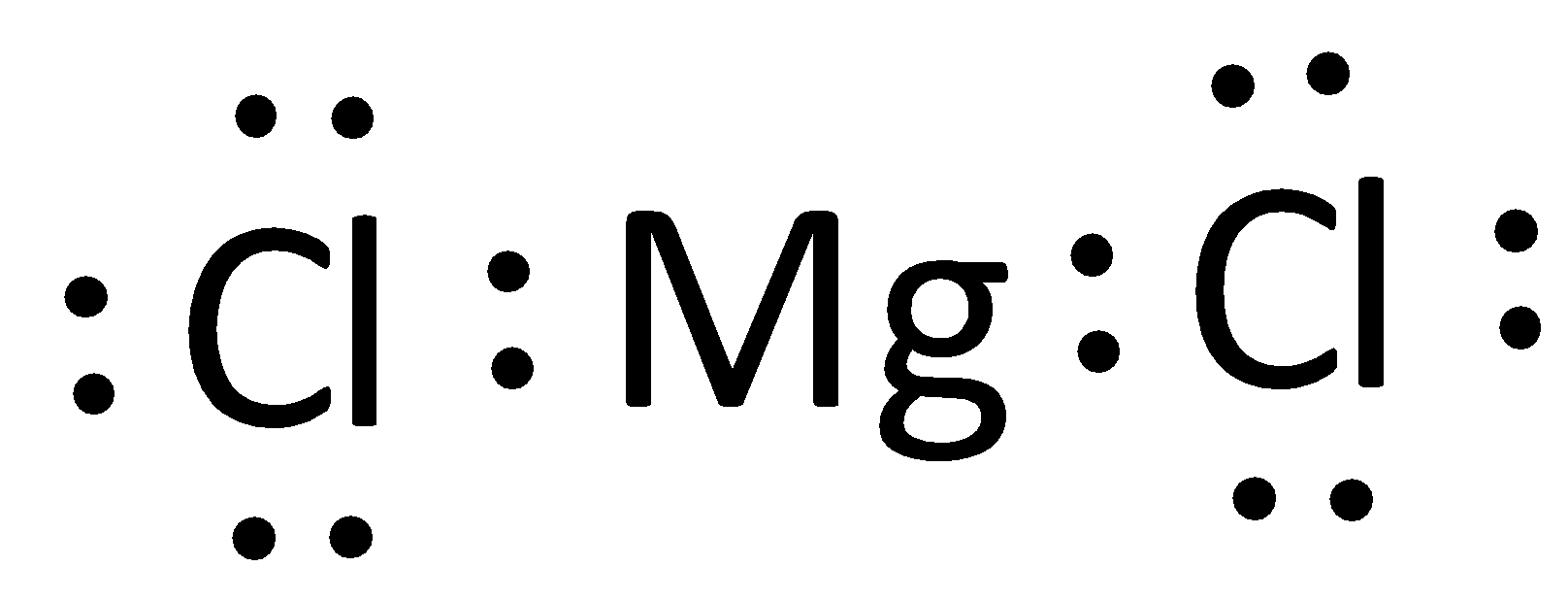
List the number of ve- for these elements
Strontium, Sodium, Neon, Boron, Helium,Tin, Bismuth, Astatine, and Selenium.
Na 1, He 2, B 3, Sn 4, Bi 5, Se 6, At 7, Ne 8
FeS2 is commonly known for having a metallic shine and a high melting point, it is a ?
Ionic Bond
Rubies are a naturally forming crystal, which formula represents this?
SiO2 C6H12O6
Al2O3 CO2
Al2O3
C2 is perfect for conducting heat and electricity.
T/F
False
How many valence electrons are needed in the outer shell to be seen as stable? What are the exceptions?
8 with the exception of H and He who need 2
List the energy levels for these elements
Og, Li, H, V, Au, Te, Al
7, 2, 1, 4, 6, 5, 3.
Copper (bonded), when in water can still conduct electricity and heat making it,
Ionic
Give a good example of an Ionic Bond.
I'm sure you got it right !
Pick the covalent bond(s)
Na2O PCl3
NH4 Mg2Cl3
NH4 and PCl3
Make Lewis Dot Diagrams for Br and Ga. Then make them stable. Give the chemical formula and oxidation #'s for the elements given.
Br-1 Ga+3
GaBr3