What is the overall direction for Atomic Radius?
Towards Francium, decreasing from left to right and decreasing up to down
What are the dots called around an element symbol?
valence electrons
Is H2O polar or nonpolar? Explain
Water is polar because its bent shape and unequal sharing of electrons between oxygen and hydrogen create a positive and negative end
How many bonds does oxygen want?
2 bonds
What is the formula for dihydrogen monoxide
H2O
What is higher in the Ionization energy: Silicon or Phosphorus?
Phosphorus
How many valence electrons does Strontium have?
2
What are the forces found on HF?
Hydrogen bonding
What is the difference between covalent and ionic bonding?
covalent bonds: nonmetal and nonmetal and sharing electrons
ionic bonds: metal and nonmetal and transferring electrons
What is the name for P5S4
pentaphosphorus tetrasilicide
What element has the highest electronegativity?
Fluorine
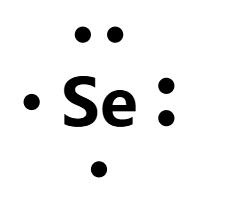
Draw the Lewis structure for Selenium
Can you explain why larger molecules with similar numbers of electrons can have different boiling points, and how London dispersion forces relate to this?
because they have more electrons that are farther from the nucleus, making their electron clouds more polarizable
Draw the structure for HF
Ms. Berg will draw it
What is the name of the formula Copper (II) Sulfate
CuSO₄
Put these elements in order of Increasing Metallic Character
O, V, Y, Ga
O, Ga, V, Y
Draw the Lewis structure for Ca 2+
Ms. Berg will draw on board
Why does the boiling point of halogens (F₂, Cl₂, Br₂, I₂) increase with molecular weight, and what intermolecular forces are responsible?
larger, heavier molecules have more electrons and a larger surface area, leading to stronger London dispersion forces
Draw the lewis structure for Calcium and fluorine
Ms. berg will draw it
What is the name of the formula Zn(CN)2
zinc cyanide
Arrange the following atoms in order of decreasing atomic radius:
Ag, Ge, S, Cs
Cs, Ag, G, S
Draw the lewis structure for H2O
Ms. Berg will draw on board
Is SO2 polar or nonpolar, and what IMFs are present?
Polar, dipole-dipole
Draw the lewis structure for aluminum and phosporus
ms. berg will draw it.
What is the formula for
Iron (II) Nitrite?
Fe(NO₂)₂