ammonium
write the chemical formula for carbon dioxide
CO2
VSEPR is an acronym that means
Valence Shell Electron Pair Repulsion
lewis structures only show these types of electrons
valence electrons
intermolecular forces exist between ________ compounds.
covalent
name this compound:
N2O4
dinitrogen tetroxide
Write the chemical formula for cobalt (II) nitrate
Co(NO3)2
this molecular geometry consists of 3 bonded atoms around a central atom and has a bond angle of 120°
trigonal planar
draw the Lewis structure for water

order the 3 intermolecular forces we learned about from strongest to weakest
hydrogen bonding, dipole-dipole, London Dispersion Forces
the compound phosphorus pentoxide contains this many oxygen atoms
5
write the chemical formula for trinitrogen heptoxide
N3O7
what shape is this molecule?

trigonal pyramidal
the following diagram shows the transfer of electrons to form what ionic compound?
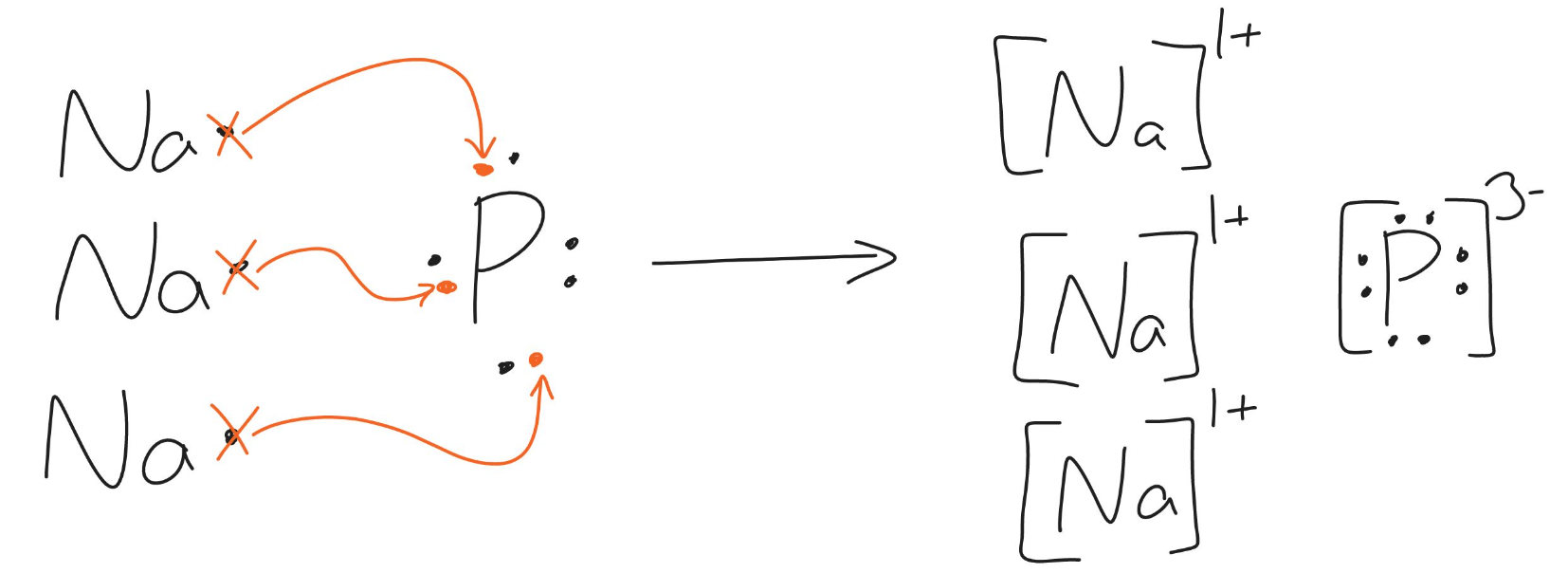
Sodium Phosphide (Na3P)
fluorine, oxygen, and nitrogen are 3 elements that are necessary for the formation of these IMFs
hydrogen bonds
name this compound: Sn(CO3)2
Tin (IV) Carbonate
write the chemical formula potassium phosphide
K3P
classify this molecule as polar or nonpolar:

polar
draw the Lewis structure for propane (C3H8)

In hydrogen bonding, H is always what kind of charge?
positive
name this compound: Na3PO4
sodium phosphate
What would the charge be on the acetate anion (C2H3O2) in NaC2H3O2?
-1
Methane (CH4) has which molecular geometry?
tetrahedral
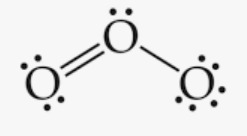
draw the lewis structure for ozone (O3) and indicate its molecular shape
bent
What Law explains both ionic bonding and dipole interactions? (it's named after someone)
Coulomb's Law