In an exothermic reaction, heat is _____________ into the surroundings
Released
What is a criteria?
Something a design requires to be successful
Does energy move from hot to cold or cold to hot?
Hot to cold
What's the first step of the engineering design process?
Identify a problem
How many oxygens are on the reactant side of this equation?
2H10O6 ----> 4H5O + 6O2
12
An endothermic reaction _______________ heat and feels ________________.
An endothermic reaction absorbs heat and feels cold.
Something limiting your design
If energy is transferring between two systems, when will energy transfer finally stop?
When both objects or systems are the same temp
What is the last step of the engineering design process
Communicate results
How many hydrogens are on the product side of this reaction?
2H10O6 ----> 4H5O + 6O2
20
Provide two examples of an exothermic reaction
MRE, handwarmer, root killer reaction, elephant toothpaste, etc
Provide three examples of criteria one might have for a new car design.
Fast, modern features, good speaker system, quiet, environmentally-friendly, etc.
Define temperature
What is the next step right after you build a prototype?
Test the prototype
Is this chemical equation balanced?
2H10O6 ----> 4H5O + 6O2
Yes
Provide two examples of an endothermic reaction
Ice pack, baking soda & vinegar, photosynthesis, frying an egg, etc
Provide three common examples of constraints.
Budget
Time
Materials
What is the relationship between the amount of reactants and the amount of heat produced by an exothermic reaction?
The amount of reactants determine the amount of heat produced
Why is the engineering design process sometimes modeled as a circle?
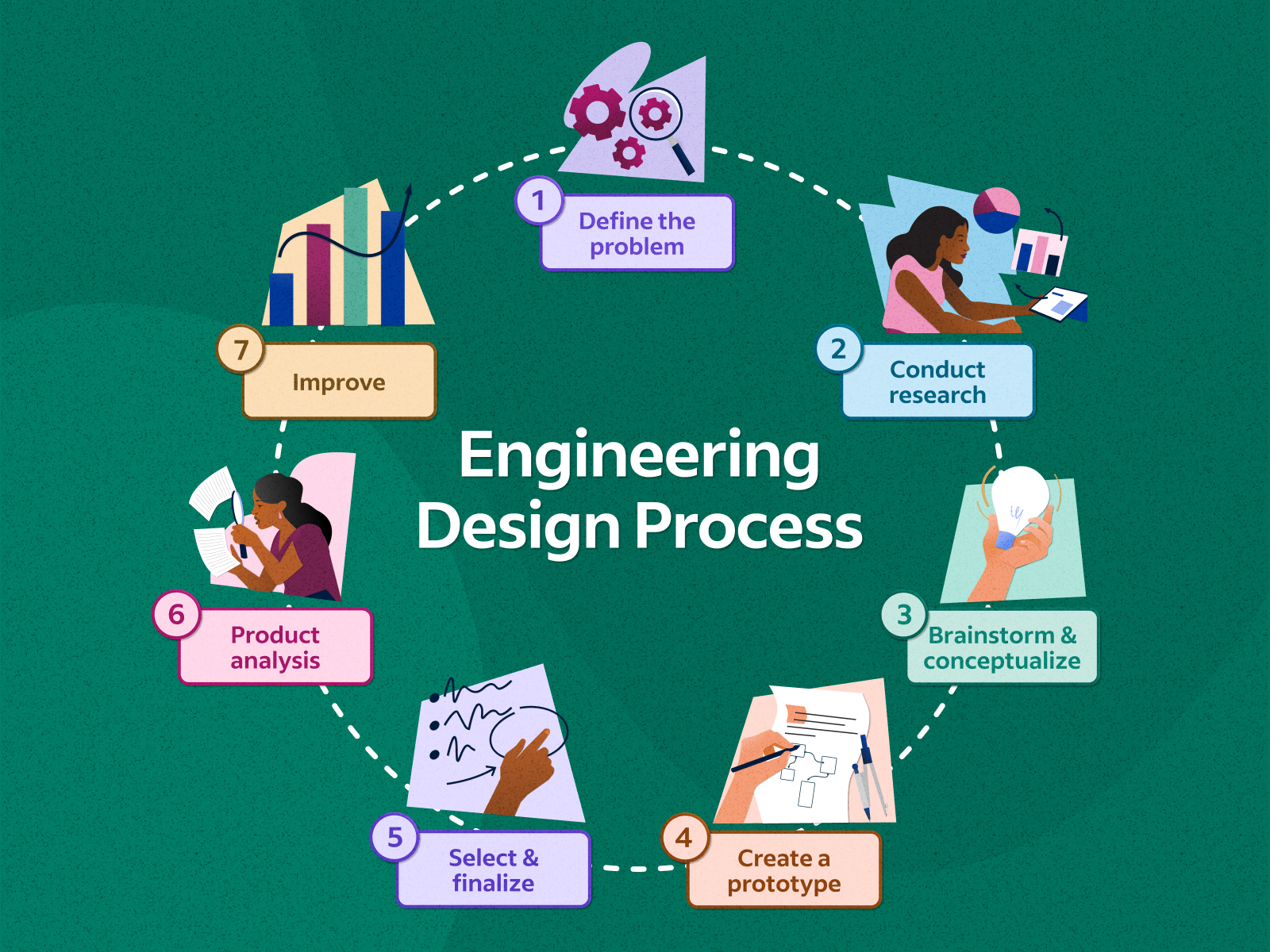
Engineers frequently repeat steps like testing and evaluating to refine a solution, which creates a cycle of improvement rather than a one-way street from start to finish.
Balance the following equation:
H2 + O2 ---> H2O
2H2 + O2 ---> 2H2O
Why does an endothermic reaction feel cold if it's absorbing heat?
It absorbs heat because it's colder than it's surroundings (EXAMPLE: ice cube on a summer day)
Pretend you are a client asking for a custom soccer uniform. Provide two criteria and one constraint for the designer.
Example criteria: must be green, comfortable, warm, etc.
Example constraints: must cost less than $50, must be completed by next Wednesday, must be made of 100% cotton.
Why might a cup of water appear to have a greater volume when it's hot compared to when it's cold?
Particles are farther apart when they're hot, increasing the overall volume of the liquid
What is a prototype?
an early model or sample of an idea that shows what the final product or project will look like and how it will work
Balance the following equation:
K + 2MgBr ---> KBr + Mg
2K + 2MgBr ---> 2KBr + 2Mg