The name of H2O
What is water
The formula for potassium oxide
What is K2O
The name for HCl
What is hydrochloric acid
The total number of electrons available for bonding in PCl3
What is 26
The type of bond likely to form between carbon and sulfur
What is nonpolar-covalent
What is the name for Ca3N2
What is calcium nitride
The formula for ammonium
What is NH4+
The formula for calcium phosphate
What is Ca3(PO4)2
The Lewis Structure for N2

Polar or nonpolar: PCl3
What would be its strongest IMF
What is polar, dipole-dipole
What is the name of H3PO3
What is phosphorous acid
The formula for acetic acid
What is HC2H3O2
The formula for zinc hydroxide
What is Zn(OH)2
The Lewis Structure for C3H8

The molecular shape of CO2, its bond angle, and the total sigma and pi bonds it contains.
What is linear, 180, and 2 sigma 2 pi
The name for P4O10
What is tetraphosphorus decoxide
The formula for triphosphorus pentasulfide
What is P3S5
The name for C9S7
What is nonacarbon heptasulfide
The Lewis structure for HCN

List all the intermolecular forces that HCN will contain, its shape, bond angle, and total sigma and pi bonds it contains.
What is dipole-dipole and LDF, linear, 180, 2 sigma 2 pi
The name for Mn(Cr2O7)2
What is Manganese (IV) Dichromate
The formula for Iron (II) phosphate
What is Fe3(PO4)2
The formula for tin (IV) sulfite
What is Sn(SO3)2
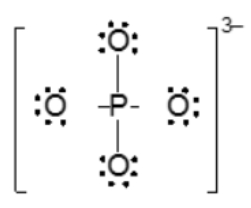
The Lewis Structure for PO43-

Determine whether PO33- is polar or nonpolar, what its shape would be, its bond angle, total sigma and pi bonds it contains, and the IMFs it would contain.
Polar, trigonal pyramidal, 109.5, 3 sigma 0 pi, and dipole-dipole and LDF