What is the role of an enzyme in a chemical reaction?
It lowers the activation energy needed for the reaction to proceed.
What does ATP stand for?
Adenosine triphosphate.
Where in the cell does glycolysis occur?
In the cytoplasm.
What organelle is the site of photosynthesis?
The chloroplast.
Which molecule is both a product of photosynthesis and a reactant in cellular respiration?
Glucose.
What happens to an enzyme's activity if the temperature or pH is too far from its optimum?
The enzyme may denature, losing its functional shape and activity.
Which bond in ATP is broken to release energy?
he bond between the second and third phosphate groups.
What are the products of glycolysis?
2 pyruvate, 2 ATP (net), and 2 NADH.
What are the two stages of photosynthesis?
The light-dependent reactions and the Calvin cycle (light-independent reactions).
How are mitochondria and chloroplasts structurally similar?
Both have a double membrane and internal membrane systems; both generate ATP using chemiosmosis.
Describe how competitive inhibition differs from noncompetitive inhibition.
Competitive inhibitors bind to the active site; noncompetitive inhibitors bind elsewhere, changing the enzyme's shape.
Describe how ATP is regenerated in the cell.
By adding a phosphate group to ADP during cellular respiration, especially in oxidative phosphorylation.
What is the main function of the electron transport chain in cellular respiration?
To create a proton gradient used to make ATP via chemiosmosis.
What is the role of water in the light-dependent reactions?
It is split to provide electrons, protons, and O₂.
ompare the role of NADH in cellular respiration to the role of NADPH in photosynthesis.
NADH carries electrons to the mitochondrial electron transport chain; NADPH provides electrons for carbon fixation in the Calvin cycle.
A mutation changes an enzyme's active site. Predict and explain the effect on the enzyme’s function.
The enzyme may lose its ability to bind the substrate effectively, reducing or eliminating its catalytic activity.
A chemical reaction in a cell has a ΔG of +3.5 kcal/mol. ATP hydrolysis has a ΔG of -7.3 kcal/mol. Explain how ATP can be used to make this reaction occur in the cell.
The cell can couple the endergonic reaction (+3.5 kcal/mol) with the exergonic ATP hydrolysis (-7.3 kcal/mol). The combined ΔG is negative (-3.8 kcal/mol), making the overall reaction spontaneous. This is how cells use ATP to power reactions that wouldn’t occur on their own.
Why is oxygen required for aerobic respiration?
It acts as the final electron acceptor in the electron transport chain.
Where do the Calvin cycle reactions occur, and what is their main product?
In the stroma; they produce G3P, a 3-carbon sugar.
f a plant is exposed to a toxin that blocks ATP synthase in the chloroplast, what will happen to the Calvin cycle and why?
The Calvin cycle will stop because ATP is required to convert 3-PGA to G3P.
The graph below shows the rate of an enzyme-catalyzed reaction at various temperatures. Analyze the graph and explain the observed trends. Then, draw a model of the enzyme's active site and indicate how its structure might change beyond the enzyme’s optimal temperature.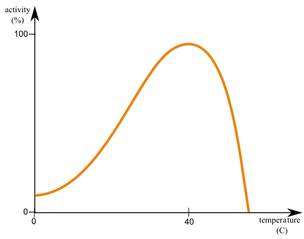
The graph shows that enzyme activity increases with temperature up to the enzyme’s optimal temperature (e.g., 37°C), where molecular collisions are more frequent. Beyond this point, the rate sharply declines due to denaturation. The heat disrupts hydrogen bonds and other interactions maintaining the enzyme’s tertiary structure, deforming the active site.
Many enzymes require ATP to function, even if they aren’t making ATP. Explain how ATP helps these enzymes drive chemical changes, using the idea of coupling.
ATP helps enzymes by transferring a phosphate group to a reactant or the enzyme itself. This added phosphate raises the energy of the molecule, making it more likely to react. This is an example of energy coupling—using ATP’s energy to make other reactions happen.
Compare the ATP yield of aerobic vs. anaerobic respiration.
Aerobic respiration yields about 30–32 ATP per glucose; anaerobic yields only 2 ATP per glucose.
Explain how the proton gradient is established in the chloroplast and how it drives ATP production.
Protons are pumped into the thylakoid lumen during the electron transport chain; they flow back through ATP synthase to generate ATP.
Explain how the processes of photosynthesis and cellular respiration form a cycle of matter and energy in a plant cell. Why is it incorrect to say that one process simply “reverses” the other?
Photosynthesis converts light energy into chemical energy by fixing carbon dioxide into glucose and releasing oxygen. Cellular respiration breaks down glucose in the presence of oxygen to produce ATP, releasing carbon dioxide and water. While the overall reactants and products appear reversed, the processes occur in different organelles, use different energy sources (light vs. chemical), and involve different pathways. Photosynthesis stores energy; respiration releases it. They are complementary but not exact opposites.