The relative ability of an atom to attract electrons to itself.
What is electronegativity?
VSEPR stands for this theory used to determine molecular shape.
What is Valence Shell Electron Pair Repulsion Theory?
Without polar bonds, a compound is only capable of this type of intermolecular force.
What are London dispersion forces?
A difference in electronegativity between 0.4 and 1.7 indicates this type of covalent bond.
What is polar covalent?
The number of domains, including lone electron pairs and bonding electron pairs, determines this.
What is electron pair geometry?
The presence of H bonded directly to a strongly electronegative atom like N, O, or F makes a molecule capable of this intermolecular force.
What is hydrogen bonding?
A bond is considered nonpolar if the electronegativities of participating atoms is less than this value.
What is 0.4?
The shape of this molecule, SF6
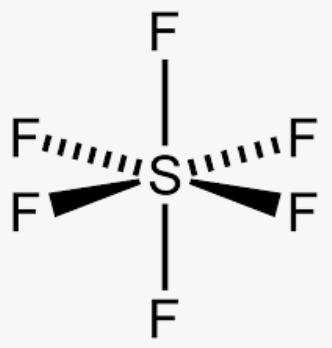
What is octahedral?
For dipole-dipole forces to occur, molecules must possess this.
What is a net dipole/permanent dipole/be polar molecules?
A polar molecule is identified by these two features.
What is a polar bond and a net dipole moment (or shape that is not symmetric, uneven distribution of the polar bonds)?
This thirst-quenching substance is a common example of bent molecule geometry.
What is water?
Considering the strengths of interactions between molecules, ion-dipole forces hold this distinction.
What are the STRONGEST interactions?
PF3 is a good example.
What is a polar molecule?
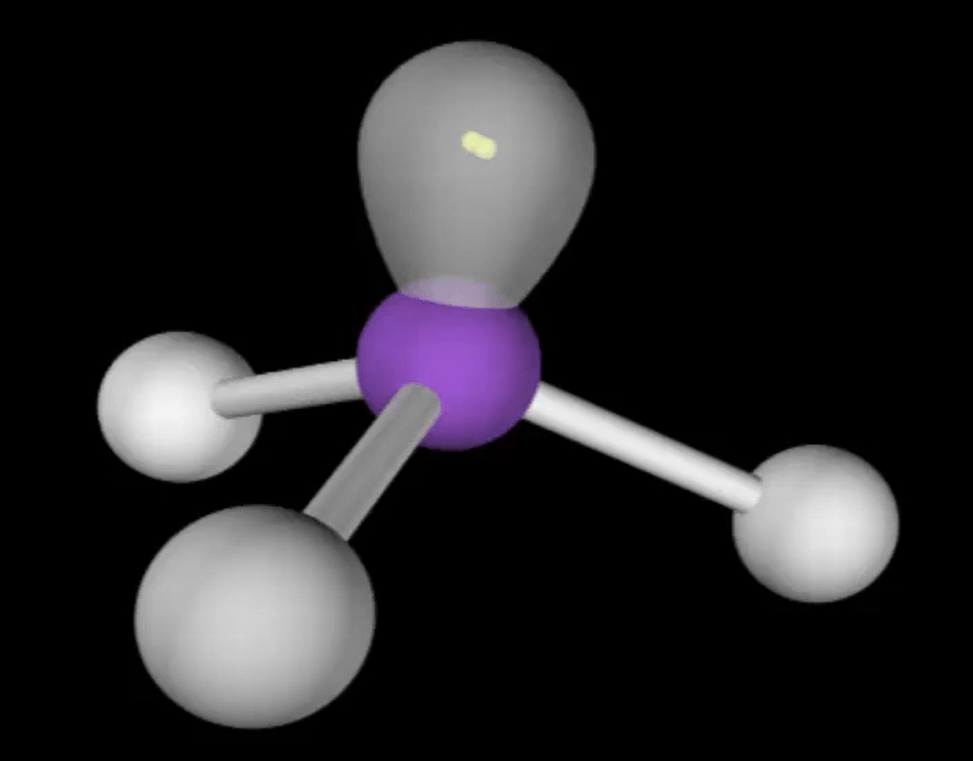
DAILY DOUBLE!
Steric number 2, steric number 5 (with 3 lone pairs), and steric number 6 (with 4 lone pairs) have this shape in common.
What is linear?
Two physical properties influenced by the strength of IMFs present.
What are physical state, solubility, melting point, boiling point, viscosity?
(Any two are acceptable.)