Which electrons are absorbing energy? 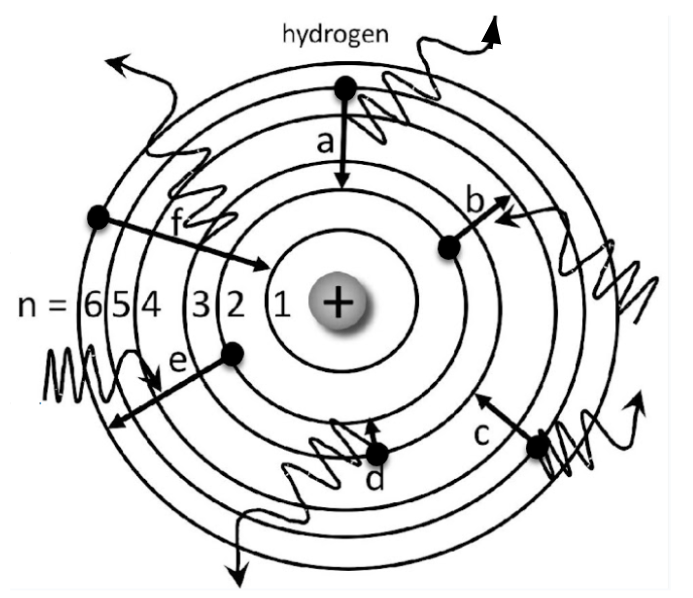
Electron B and E
What is wrong with this Bohr model?
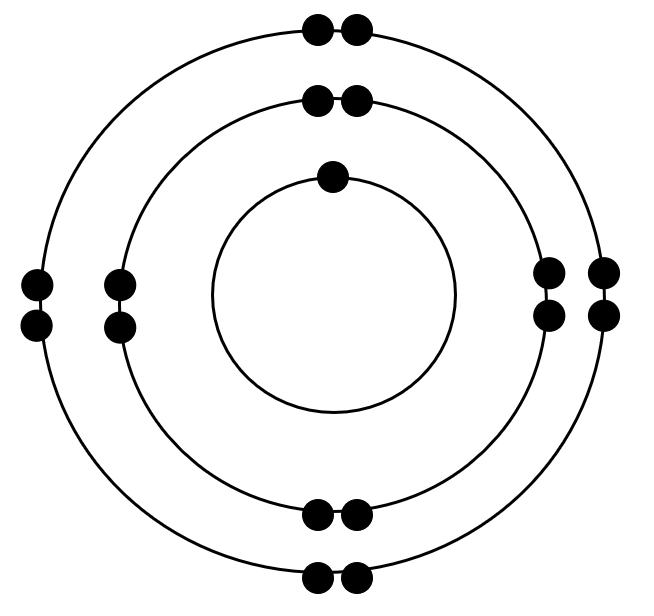
only 1 electron on first ring
For an electron to change from ground state to an excited stated it must...
Absorb energy
What subatomic particle is responsible for the spectral lines seen in emission spectra diagrams?
Electrons
True/False: A cation is a positively charged ion.
True
Which electrons start on n=5? 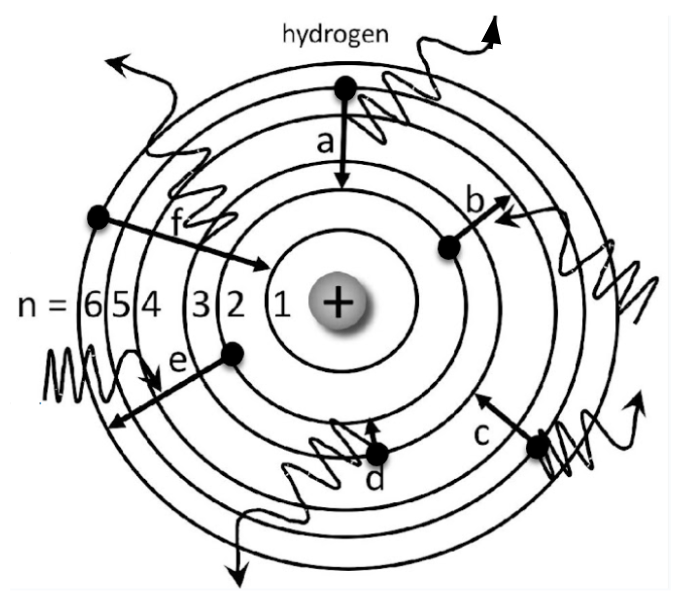
Electron A and C
Who's Bohr Model is this?
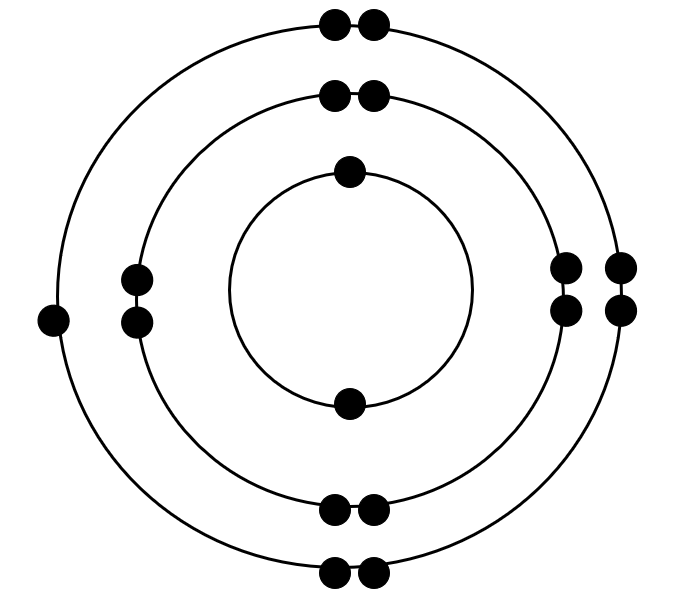
chlorine atom
When an atom has all of its electrons in the lowest energy orbitals available, the atom is...
In ground state.
Why are emission spectra called the "fingerprints" of elements?
They are all unique
How does an atom become a anion?
It gains electrons!
Which elecrton is emitting the greatest amount of energy? 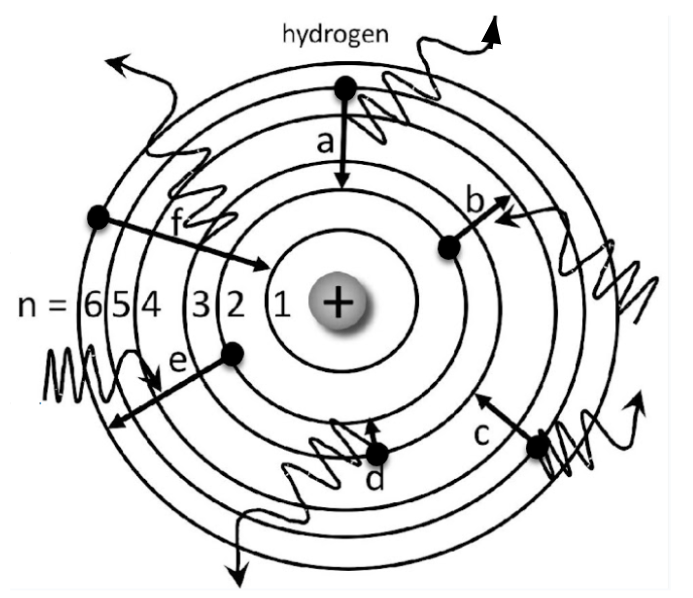
Electron F
Who's Bohr Model is this?

Phosphorous atom
True or False: The energy absorbed when an electron moves up orbitals is equal to the energy emitted when the electron jumps down orbitals.
True
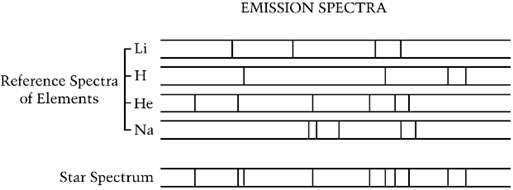 What elements are in the star spectrum?
What elements are in the star spectrum?
Hydrogen (H) and Helium (He)
Sodium (Na) forms an ion with a +1 charge. What happened to its electrons?
It lost one electron to become a cation.
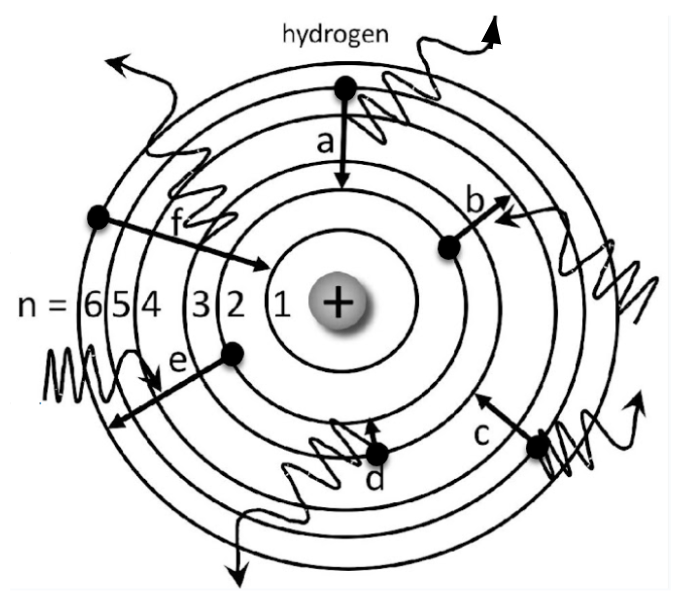 Which electron has the greatest energy absorption?
Which electron has the greatest energy absorption?
Electron E
Who's Bohr Model is this?
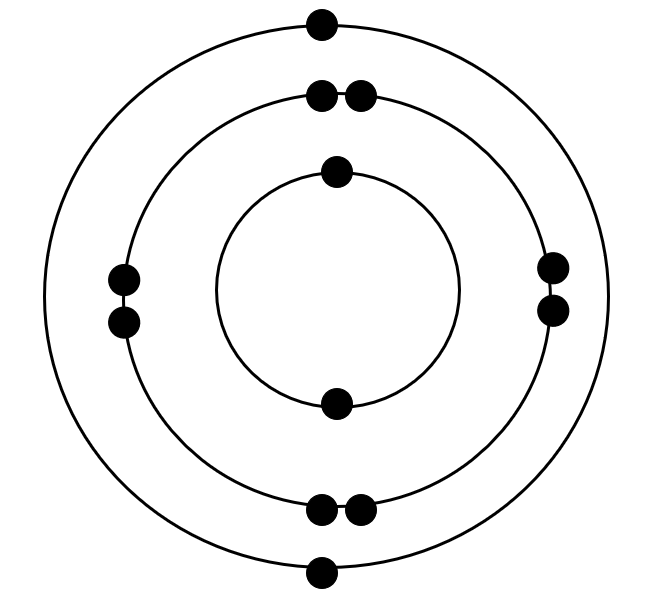
Magnesium atom
An atom that has gained energy beyond normal is said to be ...
Excited state
What elements are in the mixture?
Gas A and D
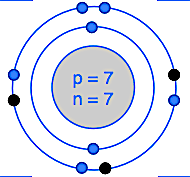
What is this element with its correct ion symbol?
N3-
Which electron is likely to emit RED light? 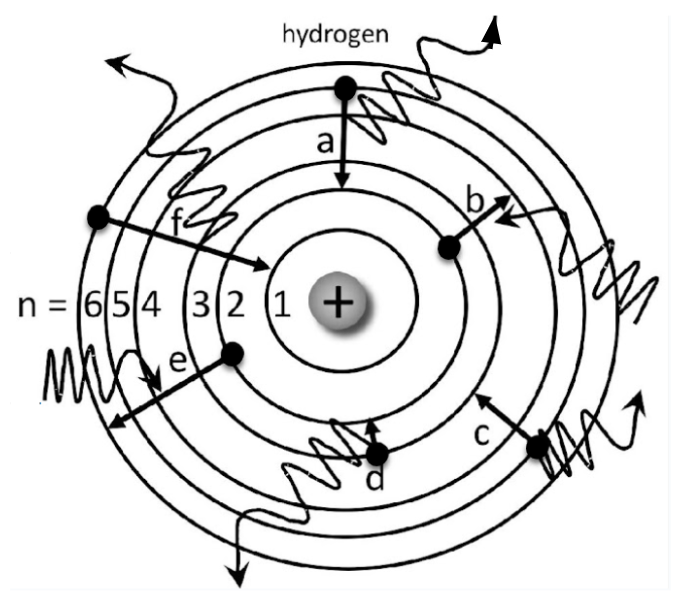
Electron D
Who's Bohr model is this and how many protons and neutrons does it have?

Sodium atom, p = 11, n=12
Compare the wavelengths ( λ ) being emittedfrom atom 1 (n=4 to n=2) and atom 2 (n=5 to n=2).
Shorter wavelength for atom 2.
Longer wavelegth for atom 1.
What is the lowest energy emission in the unknown spectra? 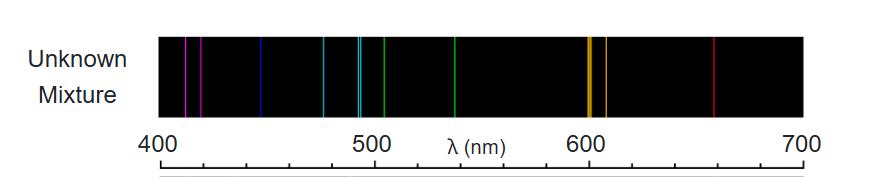
660nm (Red)
What element atom is this? Include number of protons and neutrons in answer.

Calcium ion. 20 protons & 20 neutrons.