Draw the lewis dot structure for N2. Polar or non-polar?

non-polar!
What is the bond angle associated with a linear shape?
180 degrees
How would you define polar versus nonpolar molecules?
polar- unequal sharing of electrons, difference in atom electronegativity
nonpolar-equal sharing of electrons
How do you determine what type of IMF a molecule has?
You need to determine if the molecular is polar or nonpolar and distinguish which atoms are in the molecule.

Which letter represents a phase change of liquid to gas?
D
Draw the Lewis Structure for COHF. Is it polar or non-polar?

it's polar! because it's not bonded to the same atom all the way around!
What is the VSEPR shape associated with this molecule?
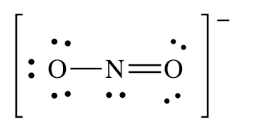
Bent
What type of IMF does dimethyl ether have between its molecules?
dipole dipole. the oxygen end is partially negative and would interact with the hydrogen end of a neighboring molecule. It would not be hydrogen bonding because C-O is not a super charged bond!
Identify and draw the IMF between BF3
LDF

What is the freezing point of the substance?
~-70C (definitely below 60 line so it is a larger negative number than 60)
Draw the lewis dot structure for PO43-
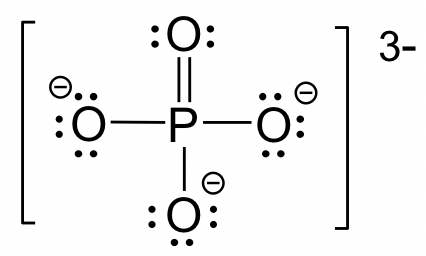
How do lone pairs on the central atom affect the bond angle? (assume the number of attachments remains the same)
The bond angle DECREASES by 2.5 degrees with the addition of each lone pair.
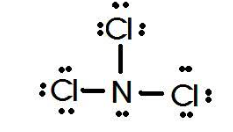
Draw the lewis structure for NCl3 and determine its polarity
Polar
Identify and draw the IMF between CH3Cl
Dipole-Dipole
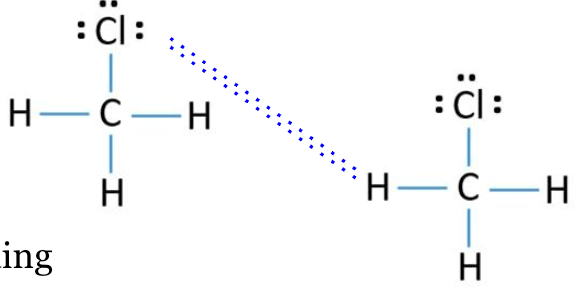
Describe what is happening during a phase change?
The intermolecular forces are being broken (the attraction BETWEEN molecules)
*No bonds are broken!
Is there anything wrong with this lewis dot structure? If so, identify the mistake(s) AND draw the correct structure!
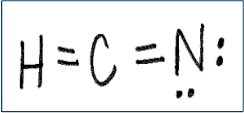
1. Too many electrons drawn (only have 10 valence electrons available)
2. Hydrogen only wants 2 electrons to be stable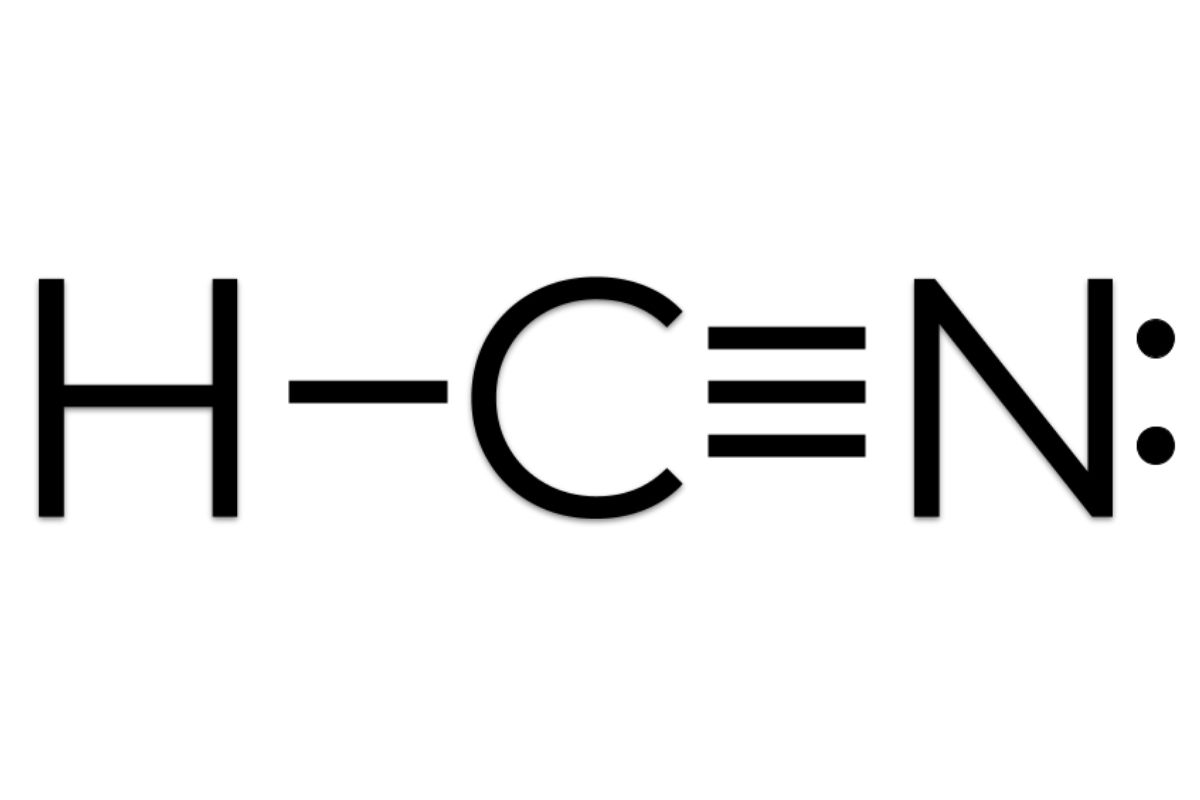
What is the molecular geometry AND bond angle associated with this molecule?
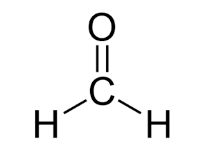
Trigonal Planar, 120 degrees
Draw the lewis structure for CO2 and determine its polarity
Nonpolar
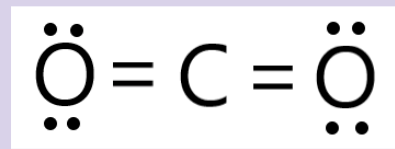
Which liquid would result in more drops on our penny: water or acetone? Explain WHY.
Water has hydrogen bonding which is a stronger IMF than dipole-dipole (acetone). A stronger IMF means stronger surface tension. It is harder to break the attraction between the molecules which would result in more liquid drops on our penny.
Which molecule will change its phase first: methanol or ethanol? Explain WHY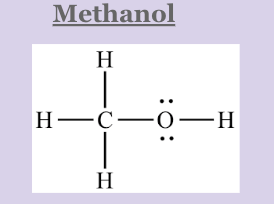
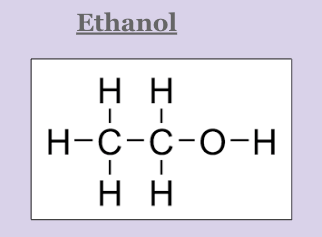
Methanol will change its phase first because even though they both exhibit hydrogen bonding (strong IMF), ethanol is a larger molecule. It will take more energy to break the IMF of the larger molecule (Ethanol)
Draw the lewis structure for CO32-
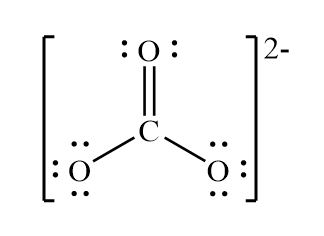
What is the molecular geometry of each central atom (left to right) associated with this molecule?
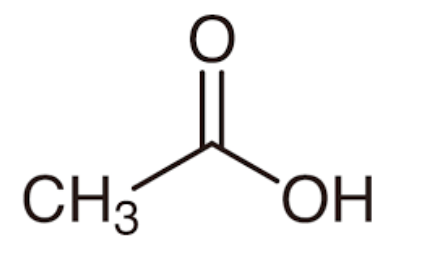
Tetrahedral, Trigonal Planar, Bent
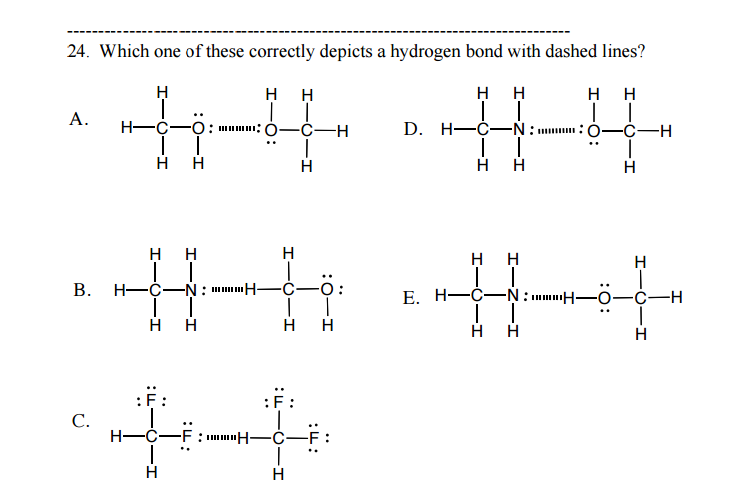
E. Only E has H-FON (bonded directly to each other!) in each molecule and the correct charged ends are interacting (negative to positive)
Why is hydrogen bonding the strongest IMF?
H-F, H-O, H-N bonds are very polar (very large electronegativity differences)

Which substance has stronger intermolecular forces?
P has stronger IMF because it has a higher MP and BP. That means it takes more energy to break the molecules apart to phase change!