What are indications a chemical change has occurred?
A. An ice cube melts
B. Steel conducting electricity
C. No color change
D. A new substance is formed
D. A new substance is formed
When sugar is added to water to make a sugar solution, a physical change occurs. According to the Law of Conservation of Mass, what prediction can be made about this physical change?
A. The sugar solution will have less mass than the sugar and water.
B. The sugar solution will have more mass than the sugar and water.
C. The sugar solution will have the same mass as the sugar and water.
D. The sugar solution will have the same volume as the water in the beaker.
C. The sugar solution will have the same mass as the sugar and water.
How does increasing temperature affect the rate of a chemical reaction?
Increasing the temperature will speed up the rate of a chemical reaction.
What is an independent variable?
An independent variable is what is being manipulated or controlled. On the x-axis of a graph.
True or False?
Laws are descriptions of phenomena, while theories are explanations of why phenomena exist.
True! Laws are descriptions of phenomena, while theories are explanations of why phenomena exist.
Is baking a cake a physical or chemical change? Why?
It is a chemical change because a new substance is formed!
Robert is hiking through the forest and writing down his observations. He steps on a branch, and it breaks into several pieces. Did a physical or chemical change occur? Did the amount of mass change?
Only a physical change occurred, and the total mass of the branch pieces is the same.
How does decreasing the temperature affect the rate of a chemical reaction?
Decreasing the temperature slows down the rate of the chemical reaction.
What is a dependent variable?
The outcome. y-axis on the graph.
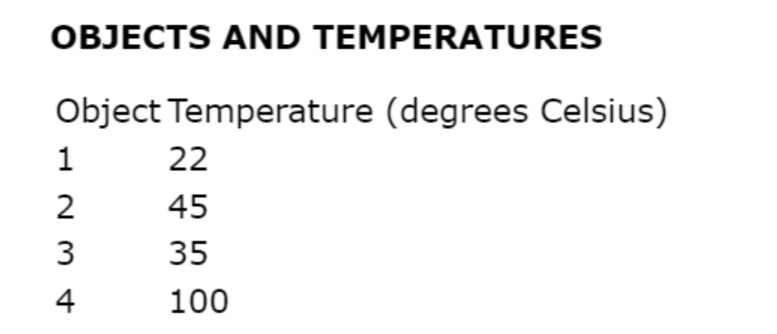
Miss Butalia conducts an experiment to determine the effect of adding salt on the boiling temperature of water. Identify the independent variable & dependent variable.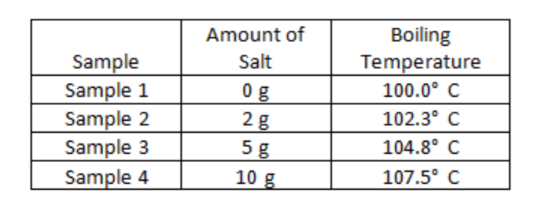
independent variable: amount of salt added
dependent variable: resulting boiling temperature
The melting of wax is a physical change, yet the burning of wax is a chemical change. What is the essential difference between a physical change and a chemical change of wax in a burning candle?
A. Melted wax is in a different phase of matter than solid wax
B. A higher temperature is needed to burn wax then to melt wax
C. Melted wax can be separated into other substances, while solid wax cannot
D. The burning of wax forms new compounds while the melting of wax does not
D. The burning of wax forms new compounds while the melting of wax does not
Which model of a chemical reaction best represents the Law of Conservation of Mass?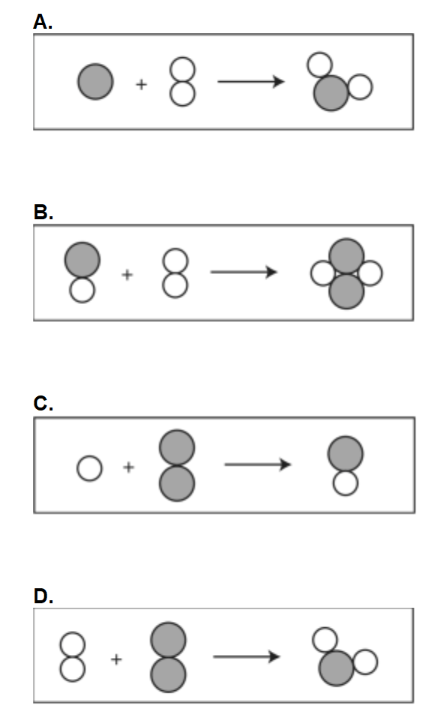
A chemist is performing a chemical reaction. Which of the following would speed up the rate of a reaction a chemist is performing?
A. Heat the beaker on a hot plate.
B. Increase the volume of the beaker.
C. Transfer both liquids in the beaker to a flask.
D. Decrease the rate at which both liquids are stirred.
A. Heat the beaker on a hot plate.
What happens when an ice cube is thrown into a fire?
The heat of the fire will be transferred to the ice, causing it to change its state.
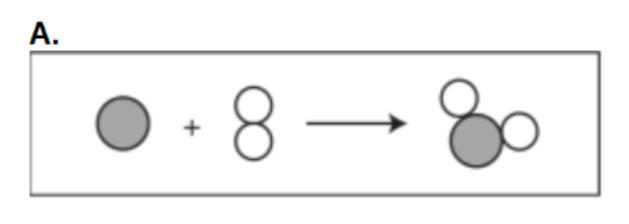
Describe how heat would flow between the following 4 objects.
Heat will flow from objects of higher temperature to lower temperature
Ethan is observing chemical and physical properties of a substance. He heats a substance and observes that the substance turns from a brown solid to a black powder. He refers to several chemistry journals that claim this represents a chemical reaction. From his observation and research, he concludes that the substance goes through a chemical change when heated. How can Ethan best defend his conclusion?
A. By demonstrating that the substance will eventually melt if the temperature continues to increase
B. By verifying that the substance is now made up of different molecules than before it was heated
C. By verifying that the substance is made up of only one type of element
D. By demonstrating that the substance is less dense after it is heated
B. By verifying that the substance is now made up of different molecules than before it was heated
What happens to the mass of the liquid in the thermometer as the level of the liquid rises?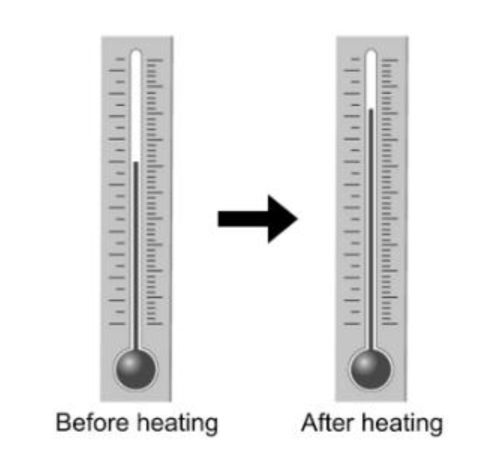
Explain why an opened bottle of milk in a refrigerator stays fresh longer than one at room temperature on a table.
*Hint: Think about how temperature affects the rate of a chemical reaction!
The lower temperature inside the refrigerator reduces the rate of the chemical reaction.
Mario takes a cold can of soda out of a refrigerator. He sets the cold can on a table in the room and leaves it there for a few hours. Describe the heat transfer.
Heat from the air will move into the soda can until both reach the same temperature.
Which statement correctly explains why this is a law, rather than a theory?
A. It has more scientific evidence supporting it.
B. It has both independent and dependent variables.
C. It has been tested with the latest laboratory balances, which are very accurate.
D. It describes a repeatedly observed relationship, but it does not offer an explanation.
D. It describes a repeatedly observed relationship, but it does not offer an explanation.
Glass shatters. Physical or chemical change? Why?
Glass shattering is a PHYSICAL CHANGE because the chemical composition remains the same; only its physical form changes. Meaning the molecules that make up the glass are still the same, just arranged differently in smaller pieces; no new substance is created.
A chemist measures the temperature at which a particular liquid boils. She makes the measurement a second time. Which of these is most likely described in this scenario?
A. repetition
B. replication
C. error analysis
D. equipment check
A. repetition
In the glowstick lab, which beaker demonstrated increasing temperature increases the rate of reaction?
A. Beaker 1 Cold
B. Beaker 2 Room Temp
C. Beaker 3 Hot
D. None of them
C. Beaker 3 Hot
Which sentence correctly explains the change that occurs when water boils and evaporates?
A. Water boiling is a physical change, and the state of the substance remains the same.
B. Water boiling is a physical change, and the mass of the substance remains the same.
C. Water boiling is a chemical change, and the state of the substance remains the same.
D. Water boiling is a chemical change, and the mass of the substance remains the same.
B. Water boiling is a physical change, and the mass of the substance remains the same.
Which investigation is an example of repetition?
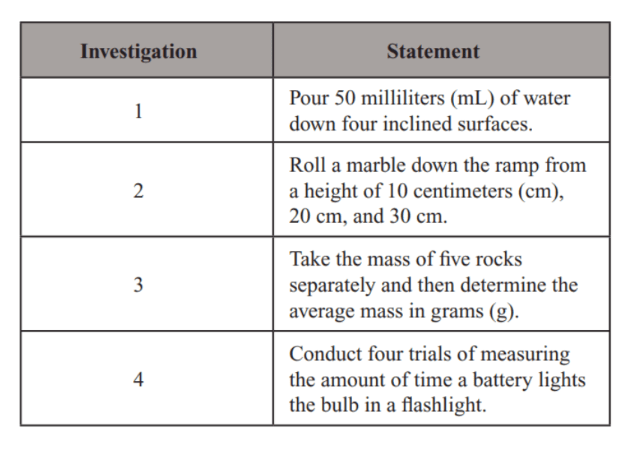
Investigation 4