What is another name for a family on the PT?
Group or Column
What is the maximum number of dots that can be placed around a chemical symbol?
8
Which rule requires that electrons in the same orbital must have opposite spins?
Pauli Exclusion principle
Name two elements that end in p4.
O, S, Se, Te, Po, and/or Lv
Name group IIA/group 2.
Alkaline Earth Metals
What element in Row 4 has this Lewis Dot Structure? 
Gallium (Ga)
Which rule requires that electrons must enter the lowest energy sublevel available first?
Aufbau principle
Write the full electron configuration for Chromium (Cr).
1s22s22p63s23p64s23d4
Name group VIIIA or Group 18
Noble Gases
What element in Row 3 has this Lewis Dot Structure?
Phosphorus (P)
Which rule requires that electrons must enter a sublevel individually in each orbital before pairing up?
Hund’s Rule
Identify the following element.
1s22s22p63s23p64s23d104p65s24d9
Silver (Ag)
What family contains lithium and potassium?
Alkali Metals or Group 1/IA
All alkali metals contain how many Lewis dots around their chemical symbol?
1
Which element is shown below?
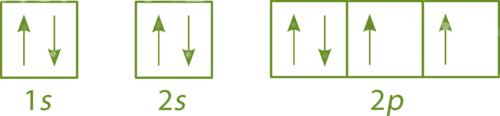
Oxygen (O)
All transition metals end with what sublevel?
d (d1, d2, d3…)
Name the family that contains the most diatomic elements.
Halogens or Group 17/VII A
Name two other elements that have the same Lewis Dot Structure as carbon.
Si, Ge, Sn, Pb, and/or Fl
How many valence electrons are shown in the element below? 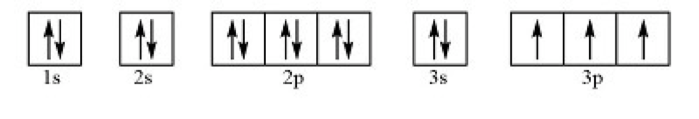
5 Valence Electrons
What is wrong with the configuration below?
1s22s22p63s23p63d104s24p64d95s2
3d and 4s as well as 4d and 5s are out of order
Name the family that contains 5 valence electrons.
Nitrogen or Group 15/VA
Which noble gas does NOT have 8 valence electrons?
Helium (He)
The element below belongs to which family?
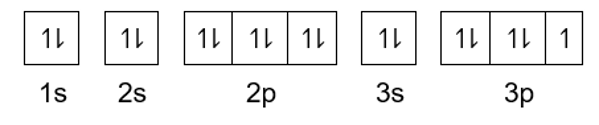
Halogens; Group 17/VIIA
Identify the three parts of the configuration below.
1s2
1 = energy level
s = sublevel
2 = number of electrons