The group number of the Alkali Metals
1
The charge/oxidation number of group 17 (the halogens)
-1
The Russian chemist who created the first periodic table
Mendeleev
The element in group two, period 4
Calcium
The trend in electronegativity as you go down a group
Electronegativity decreases as you go down a group
The group number of the Alkali Earth Metals
2
Define anion
An ion with a negative charge (formed by elements a negative oxidation number)
The number of periods on the periodic table
7
The element in group 17, period 3
Sulfur
The direction in which ionization energy increases across a period
It increases as you go from left to right
The group number of the halogens
7A / 17
Define cation
An ion with a positive charge (formed by elements with a positive oxidation number)
Do elements in the same period or group have the same number of energy levels?
Same period
The noble gas in period 5
Xenon
Atomic radius decreases as you go from left to right, and as you go up
The family name for the group 8A elements
Noble Gases
Which groups will form cations?
group 1a, 2a, 3a, sometimes 4a
The location of metals, metalloids, and nonmetals on the periodic table
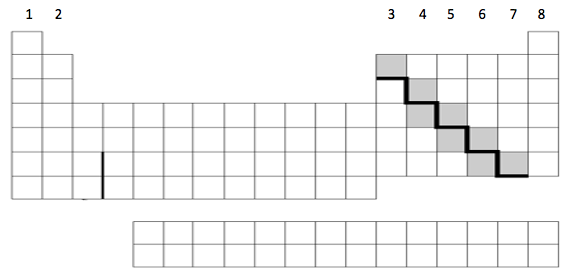
Metals to the left of the stair stepper (most of the periodic table), metalloids are shaded, and nonmetals are to the left
The group number and period number of Aluminum (Al)
3A / 13 = group, period number = 3
From which of the following atoms is it easiest to remove an electron? K, Cl, Ti, As
K. It has the lowest ionization energy. Therefore it will take the least amount of energy to remove the electron
SURPRISE QUESTION! How many atoms are in 2.5 moles of Calcium? How many grams are in 3.7 moles of Calcium?
There are 1.505 x 10^24 atoms in 2.5 moles of Calcium. (2.5 x (6.02 x 10^23))
There are 148.3 grams in 3.7 mols of Calcium (3.7 x 40.078)
The oxidation number/charge of all main group elements.
group 1a = +1
group 2a = +2
group 3a = +3
group 4a = +/- 4
group 5a = -3
group 6a = -2
group 7a = -1
Elements in the same group share the same (list 3 things)
1. chemical properties 2. valence electrons 3. oxidation numbers
Where to find the molar mass of an element.
For Fluorine (F), list its electronegativity (high or low), ionization energy (high or low), and atomic radius (high or low)
Electronegativity is high, Ionization energy is high, and Atomic Radius is low