Identify each of the following as ionic, metallic, or covalent:
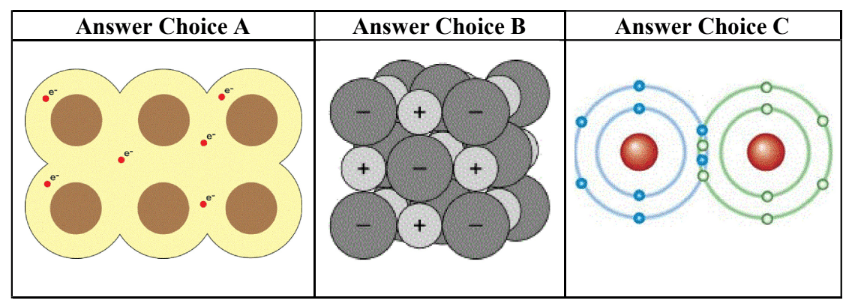
A - metallic
B - Ionic
C- Covalent
What is the chemical formula or ratio when Magnesium (Mg) bonds to Chlorine (Cl)?
ionic bond
MgCl₂ (1:2 ratio)
Identify the empirical and molecular formula for the compound shown below:

molecular: C₃H₆
empirical: CH₂
True or False: VSEPR shapes such as bent and trigonal pyramidal apply to ionic and covalent molecules.
Covalent
What is the chemical formula for diphosphorus trichloride?
P2Cl3
Ionic, Metallic, Covalent: which conducts electricity as a solution?
Ionic
They are the only electrolytes.
An unknown atom “X” bonds ionically with fluorine and makes the formula XF2. When it bonds with sulfur its formula is XS. What atom could unknown “X” be?
any atom with a +2 charge
Draw N2 AND identify the number of sigma and pi bonds.
1 sigma bond
2 pi bonds

Is this molecule polar?
nonpolar because it is symmetrical
What is the IUPAC name for P3Br5?
triphoshours pentabromide
Will shatter when hit with a hammer.
Ionic (brittle)
What is the percent composition of magnesium in Mg(OH)2?
41%
Will this molecule have the same molecular formula as it's empirical?

C3H8 cannot be reduced, so YES
What do lone pairs do the the bond angle between bonds? How do they affect the molecular shape?
Push the bonds together and make the bond angle smaller as a result of electron repulsion.
What is the IUPAC name for FeCl₂ ?
Iron (II) Chloride
Which substance should have the highest boiling point?
NaCl Fe CO2
NaCl - ionic have VERY high boiling points
Draw the lewis dot structure for ZnF2
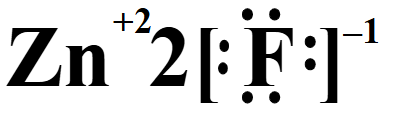
Identify the central atom. How many bonded groups and lone pairs are on the central atom?
Nitrogen is central
2 bonded groups
1 lone pair
Draw PCl₃ and identify its molecular shape, number of bonded groups, and lone pairs.
Trigonal Pyramidal, 3 bonded groups, 1 lone pair.

What is the IUPAC name for Na2SO4?
Ionic, Metallic, Covalent: Which of the following will have a small difference in Electronegativity?
Covalent
Small difference prevents the stripping of an electron. This is why covalents share electrons not transfer.
Hydrogen makes up 11% of water. If I have 50g of H2O, what mass is oxygen?
Oxygen 89%
50(.89)= 45g
Identify the number of sigma and pi bonds on the molecule below:

3 sigma and 1 pi bond
Draw the molecule for CSe₂, identify its molecular shape, and state if it is a polar molecule or nonpolar.
linear and nonpolar
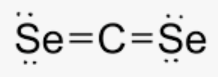
What is the IUPAC name for MnCrO₄?
Manganese (II) Chromate