Which type of compound(s) have IONIC bonds?
a. binary ionic compounds
b. ternary ionic compounds
c. binary molecular compounds
A & B
Binary Ionic Compounds & Ternary Ionic Compounds
Name the BIC: NaCl
sodium chloride
Name the TIC: Al(NO3)3
Aluminum nitrate
Write the formula for the BMC: Dihydrogen monoxide
H2O
Write formula for: sodium chloride
NaCl
Draw the Lewis Structure for the element: Li

Which type of compound(s) have COVALENT bonds?
a. binary ionic compounds
b. ternary ionic compounds
c. binary molecular compounds
C
Binary Molecular Compounds
Name the BIC: Ca3N2
calcium nitride
Name the TIC: (NH4)2SO4
Ammonium sulfate
Write the formula for the BMC: Sulfur hexafluoride
SF6
Name: (NH4)3P
ammonium phosphide
Draw the Lewis Structure for the element: Al

Classify the following compound as BIC, TIC, or BMC: (NH4)2CO3
TIC
3 or more elements
Write the formula for the BIC: Magnesium chloride
MgCl2
Write the formula for the TIC: Ammonium hydroxide
NH4OH
Name the BMC: PCl3
Phosphorus trichloride
Write formula for: ammonium carbonate
(NH4)2(CO3)
Draw the Lewis Dot Structure for the element: P
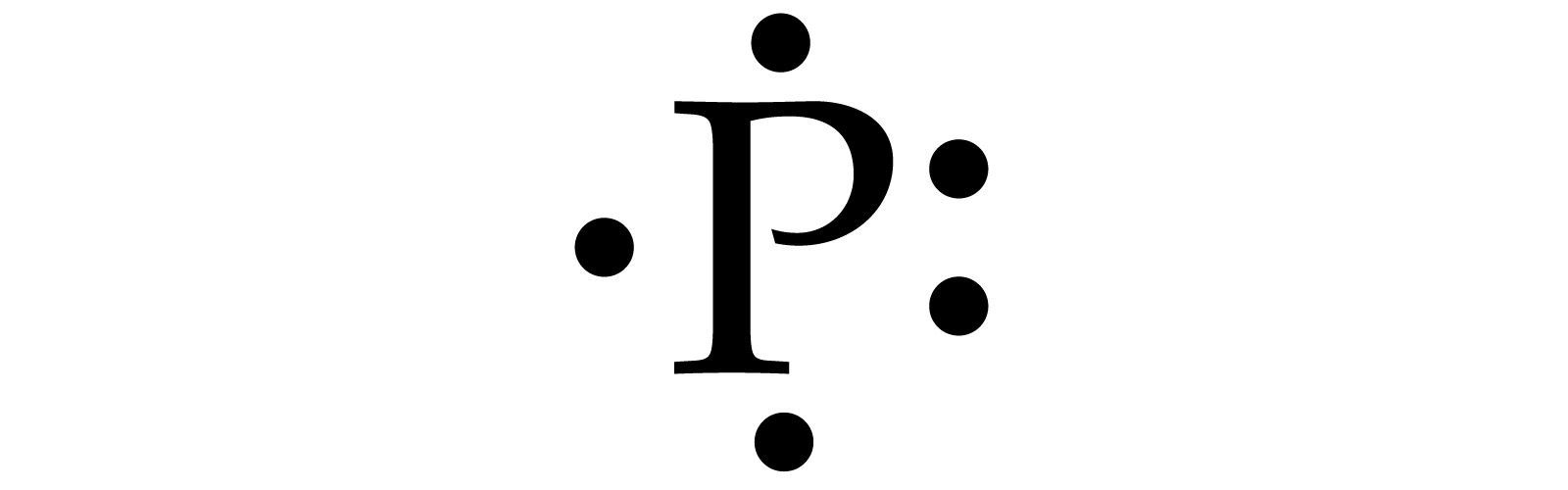
Classify the following compound as BIC, TIC, or BMC: Ca3N2
BIC
2 elements: 1 metal + 1 nonmetal
Write the formula for the BIC: Lithium phosphide
Li3P
Write the formula for the TIC: Magnesium acetate
Mg(C2H3O2)2
Name the BMC: P2O5
Diphosphorus pentoxide
Name: Br2O7
dibromine heptaoxide
Draw the Lewis Structure for the element: H

Classify the following compound as BIC, TIC, or BMC: NH3
BMC
2 elements: 1 nonmetal + 1 nonmetal
Write the formula for the BIC: Aluminum nitride
AlN
Write the formula for the TIC: Ammonium pyrophosphate
(NH4)4P2O7
Name the BMC: N2O
Dinitrogen monoxide
Write formula for: ammonium hydrogen phosphate
(NH4)3(HPO4)
Draw the Lewis Dot Structure for the element: He