How many atoms of Fe are in 0.5 moles of Fe?
3.01 x 1023
What is the empirical formula of C6H12O6?
CH2O
How many electrons are in a cation of K+?
20
Consider the following reaction:
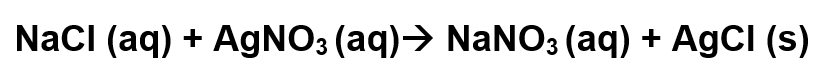
What does the (aq) mean?
Aqueous - dissolved in water
How many moles of Fe atoms are in 1.24 x1024 atoms of Fe?
2
What is the percent composition of hydrogen in water?
Your answer must have 3 sig figs and correct units.
11.1%
What family does fluorine belong to?
Halogens
Consider the following reaction:
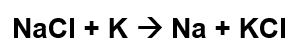
What type of reaction is this?
Single replacement
What is the molar mass of PH3?
Your answer must have 3 sig figs and correct units.
34.0 g/mol
TNT has a molecular formula of C7H5N3O6. What is the %C for this compound?
37.0%
Which of the following compounds will have the highest boiling points?
CO2, H2O, LiCl, C6H12O6
LiCl
Consider the following unbalanced reaction:
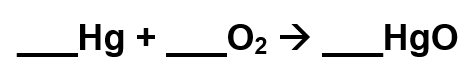
What is the coefficient for mercury when this reaction is balanced?
2
How many moles of NH3 are in 10.0 g of NH3?
Your answer must have 3 sig figs and correct units.
0.588 moles NH3
Which compound has the largest percent composition by mass of chlorine?
HCl, LiCl, KCl , FrCl
HCl
How much of an 80. g sample of 60Co remains after decaying for 15.8 years?
10. g
Consider the following unbalanced reaction:
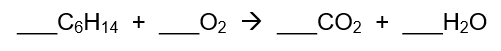
What is the coefficient for carbon dioxide when this reaction is balanced?
19
How many water molecules are in 2.0 g of water molecules?
Your answer must have 3 sig figs and correct units.
6.69 x1022 water molecules
Which of the following compounds has the smallest percent composition for hydrogen?
H2O, H2SO4, HNO3, HBr, or H2
HBr
What is the molecular shape of PF3, and is it a polar molecule?
trigonal pyramidal, yes