Covalent bonds occur between two __________.
nonmetals
What is the first step in determining the lewis structure of a molecule.
Count the # of valence electrons
AX4
AX2
AX4: tetrahedral; 109.5
AX2: linear: 180
VSEPR Theory states that electrons pairs want to __________ the repulsions by positioning themselves as far away as possible.
minimize/decrease
Covalent bonding includes the ____________ of electrons
Sharing
Which of the following is the typical physical state for a covalent compound?
a) Gas
b) Liquid
c) Solid
d) both a and b
D) both a and b
Rarely solid, if they are they are very waxy solids with low melting points
Hydrogen only needs _______ electrons to be "happy."
Do you use hybridization or a VSEPR formula to determine a molecule's geometry and angle?
VSEPR FORMULA
List of what each part of the VSPER formula represents.
A - central atom
X - # of peripheral atoms
E - # of lone pairs
Nonpolar covalent bonds share electrons ______________ while polar covalent bonds share electrons _______________, creating an atom that is slightly positive and another atom that is slightly negative.
equally; unequally
What do you call a substance that can NOT conduct electricity when dissolved in water.
nonelectrolyte
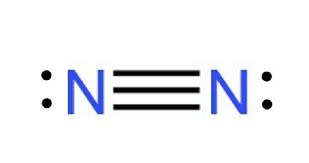
Draw the lewis structure of N2

Write out the geometry, bond angle, AND hybridization for this molecule.
AX4: tetrahedral; 109.5; sp3

Write the correct VESPR notation for this lewis structure AND the hybridization.
AX4; sp3
Which elements signify that a bond is polar covalent, (hint: there are 4... you must list all).
F, O, N, or Cl
Which two elements have the ability to expand their octet when needed?
Sulfur and Phosphorus
Draw the lewis structure for BF3

Write the correct geometry, bond angle, AND hybridization for H2O.
AX2E2: bent; 104.5; sp3
 Write the correct VSEPR notation for this lewis structure AND the hybridization
Write the correct VSEPR notation for this lewis structure AND the hybridization
AX3E1; sp3
Who is is least like to dissolve in water and why?
a) Nonpolar covalent compounds
b) Polar covalent compounds
c) Ionic compounds
d) both b & c
a) Nonpolar covalent compounds.
These compounds share electrons equally which creates an equal distribution of charges.
Which two elements, besides hydrogen, can have less than a full octet?
List the amount they need. (1 value each)
Be and B
Draw the lewis structure of chlorate (ClO3)-1
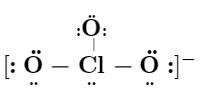
Write the correct geometry, bond angle, AND hybridization for BeCl2.
AX2: linear: 180; sp
What does VSEPR Theory predict?
Molecular geometry
Rank the following from longest to shortest bond length: double bond, single bond, triple bond.
Single bond > double bond> triple bond