Sort the following into metals, nonmetals, and metalloids: Br, Na, B, Mn, Te, O
Metal: Na & Mn
Nonmetal: Br & O
Metalloid: B & Te
Does the graph below represent a periodic trend?
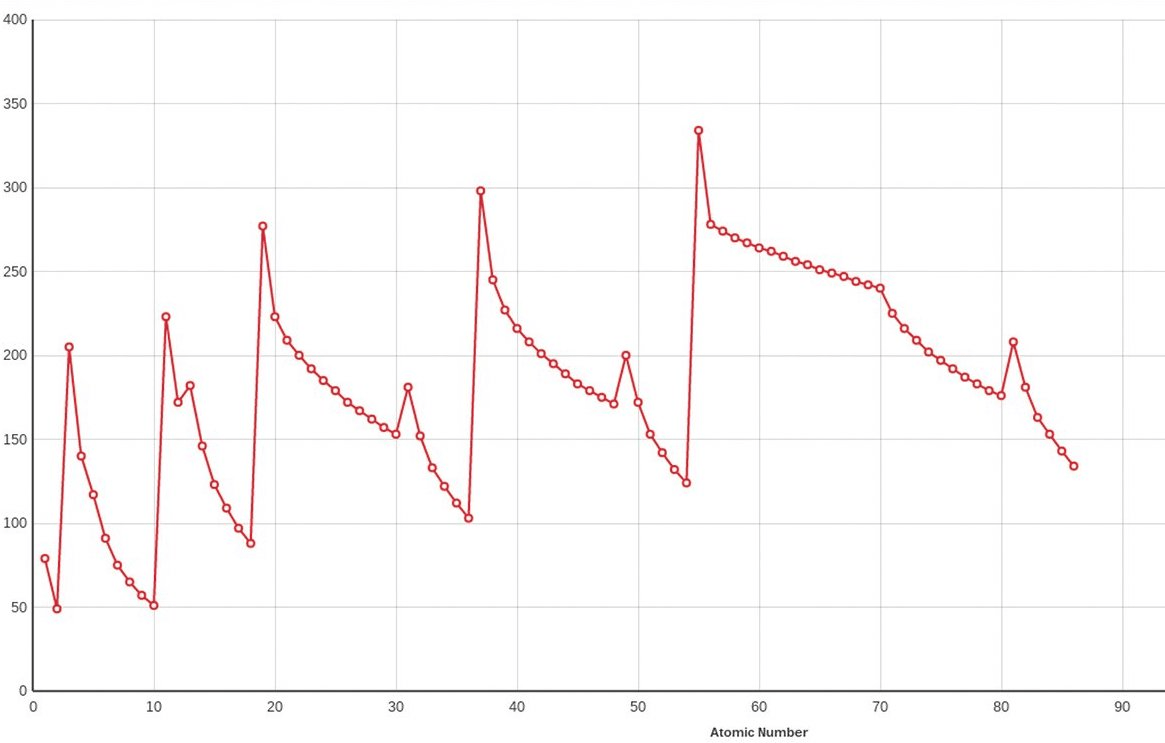
Yes (repeating pattern)
Describe hydrogen
diatomic, combustible, can lose, gain, or share its electron, extreme circumstances creates metallic properties, can create a hydrogen bond
Do the following elements have similar properties: P, Bi, Sb
Yes (all elements are in group 15, elements in the same group tend to have similar chemical and physical properties)
Where are the representative (main group) elements located?
s & p blocks
Does the graph below represent a periodic trend?
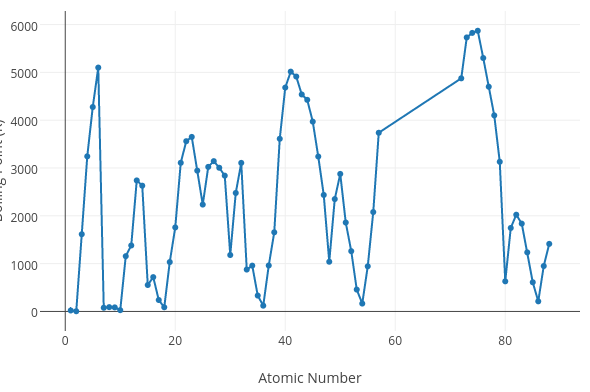
No (there is no discernible pattern)
Do the following elements have similar properties: W, Pt, Au, Hg
No (they are all in period 6, but not a part of the same group)
the nuclear charge of sulfur
16 (there are 16 protons)
The number of valence electrons in an atom of As?
As has 5 valence electrons (in group 15 or 5A)
[Ar]4s²3d104p³
Which element has a lower ionization energy?
S or Si
Si: ionization energy increases across a period, both elements are in period 3 so the element further to the left would have a lower ionization energy (also Si is further from He)
Describe Carbon
basis for all life, found in starts and the atmospheres of most planets, 3 allotropes (amorphous, graphite, and diamond), also buckminsterfullerene (C60)
Which has a larger atomic radius: Ti or Y
Y: atomic radius increases down a group and decreases across a period, we also know that helium has the smallest radius so the further an element is to He the larger it will be
alkali metals, alkaline earth, chalcogens, halogens, noble gases
(properties, valence electrons, most likely charge)
alkali metals (group 1): soft, shiny, very reactive, 1 valence electron, forms +1 charge
alkaline earth metals (group 2): shiny, silvery-white color, low melting points, 2 valence electrons, forms +2 charge
chalcogens (group 16): name refers to ores of copper (that contain oxides and sulfides and trace amounts of selenium and tellurium), 6 valence electrons, tend to form -2 ions)
halogens (group 17): high electronegativities, are diatomic elements, highly reactive, 7 valence electrons, tend to form -1 charge
noble gases (group 18): non-reactive, odorless, colorless gas, 8 valence electrons, do not form ions (due to stable electron configurations)
What element has the higher electronegativity?
N or P
N (nitrogen), electronegativity tends to decrease down a group
(the closer an atom is to fluorine on the periodic table the more electronegative the atom is)
Describe nitrogen
needed in the form of nitrates and nitrite for plant growth, used in hydrazine which is rocket fuel, Oswald process used to make nitric acid
The effective nuclear charge of sulfur
16-10 = +6
(atomic number - inner electrons = effective nuclear charge)
In what energy level are the valence electrons of zirconium and how many does it contain.
[Kr]5s24d2
2 valence electrons, 5th energy level
Which of the following elements is the most reactive: P, Ar, Br
Br, it would have the highest reactivity of the nonmetals listed because it is the closest to F after recognizing the Ar is automatically the lease reactive since it is a noble gas
Which has a larger atomic radius: Ti or Y
Y: atomic radius increases down a group and decreases across a period, we also know that helium has the smallest radius so the further an element is to He the larger it will be
Which of the following elements is the most reactive: Sr, Y, V
Sr, it would have the highest reactivity of the metals listed because it is the closest to Fr
The number of valence electrons contained by the noble gases.
8, except He which has 2
When moving left to right across a period this is increasing thus causing the atomic radius of an atom to decrease and ionization energy in increase.
Effective nuclear charge
Which metals are considered coinage metals because they are non-reactive/stable
Group 11 (Cu, Ag, Au)
The names of Ms. Zibart's Cats
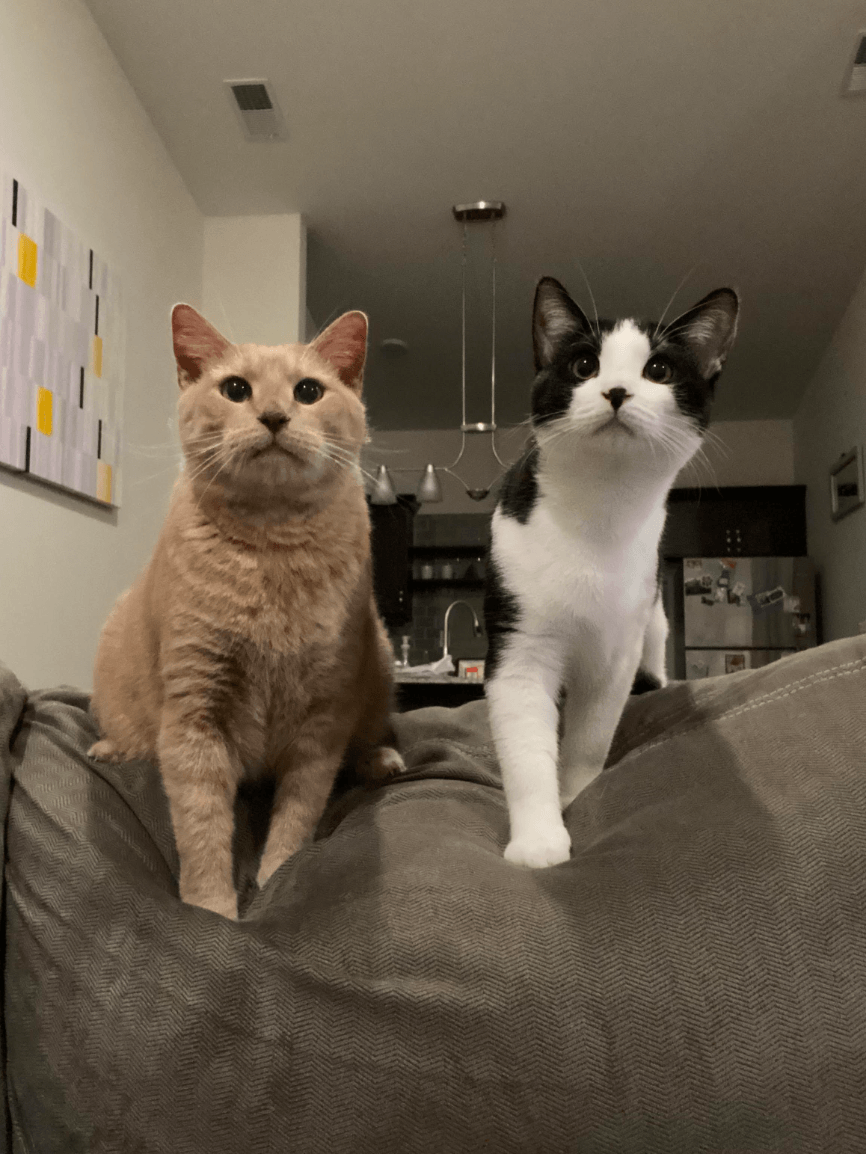
Kitty & Meeko