Where are metals mainly found on the Periodic Table of Elements?
Left, bottom excluding hydrogen.
What has a pH less than 7?
Acids
Is this chemical equation Balanced or Unbalanced: 6CO2+H2O -> C6H12O6+O2
Unbalanced
What makes water have unique properties?
Water's unique properties are due to its molecular structure. Water is a compound composed of two hydrogen atoms and one oxygen atom.
TRUE OR FALSE: Sulfur has one more proton than phosphorus
True
2H2O What is this an example of?
A compound
What has a bitter taste when eaten or drank?
Bases
What does the Law of Conservation of Matter state?
Matter cannot be created or destroyed.
What is Cohesion?
Cohesion is the force that holds molecules of a single material together.
Does this picture represent an element, compound, or mixture?

Element
True or False: 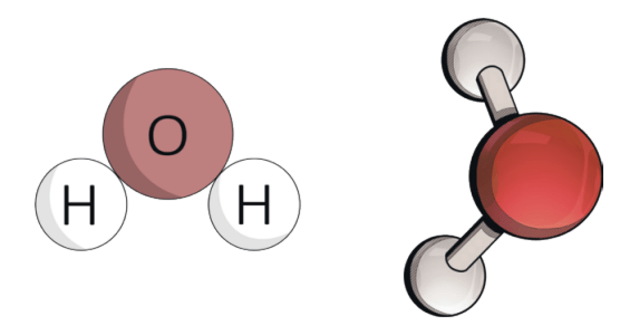 Is the picture above a element?
Is the picture above a element?
False
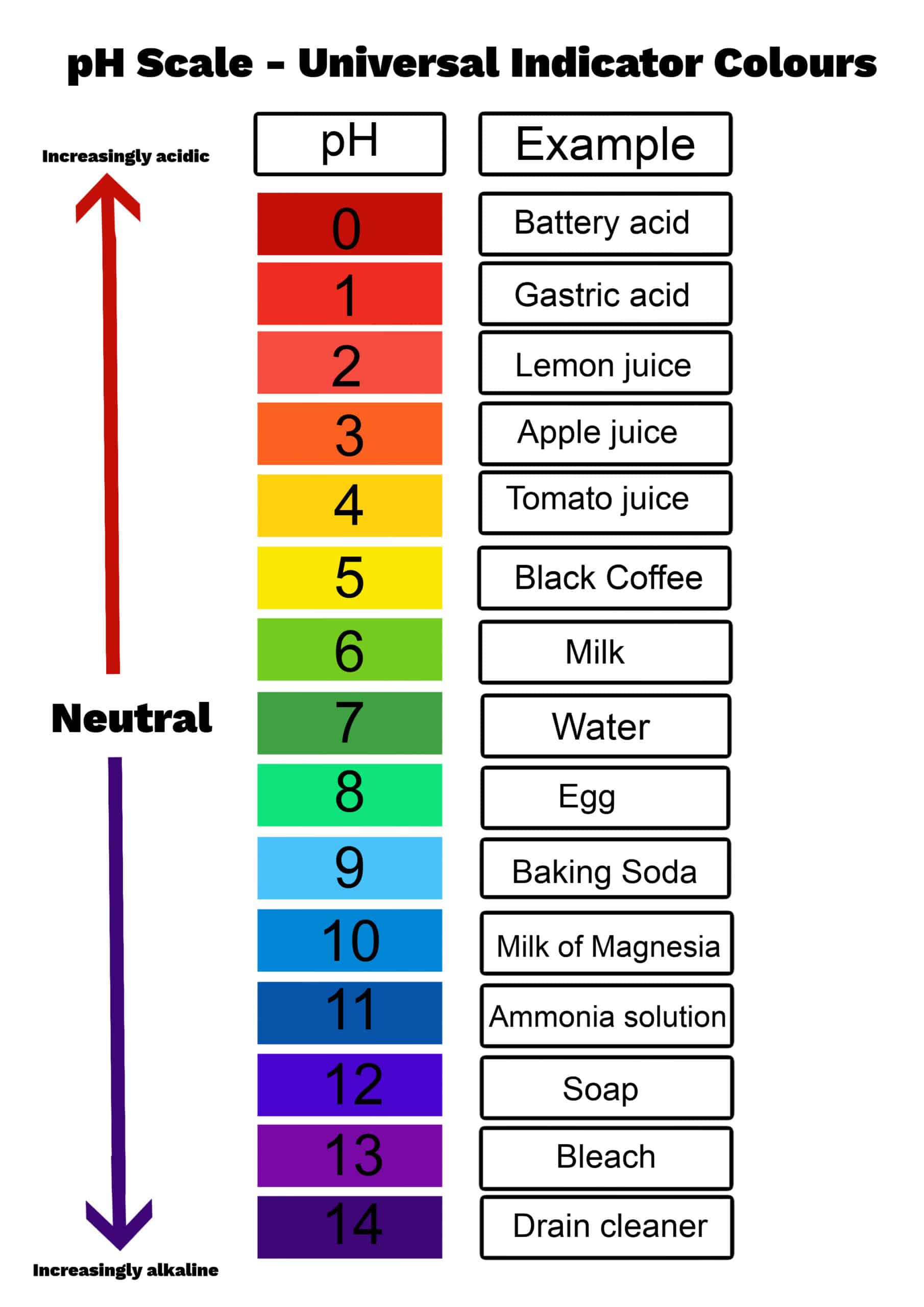 What on the pH chart has a 3 for a pH level?
What on the pH chart has a 3 for a pH level?
Apple Juice
What is a chemical reaction?
A chemical reaction is a process in which one or more substances, also called reactants, are converted to one or more different substances, known as products.
What is adhesion, give an example?
Water attracted to other materials, paper towels cleaning up water
What is a mixture? Name the two types of mixtures
A non-chemical combination of 2 or more substances, homogeneous and heterogeneous
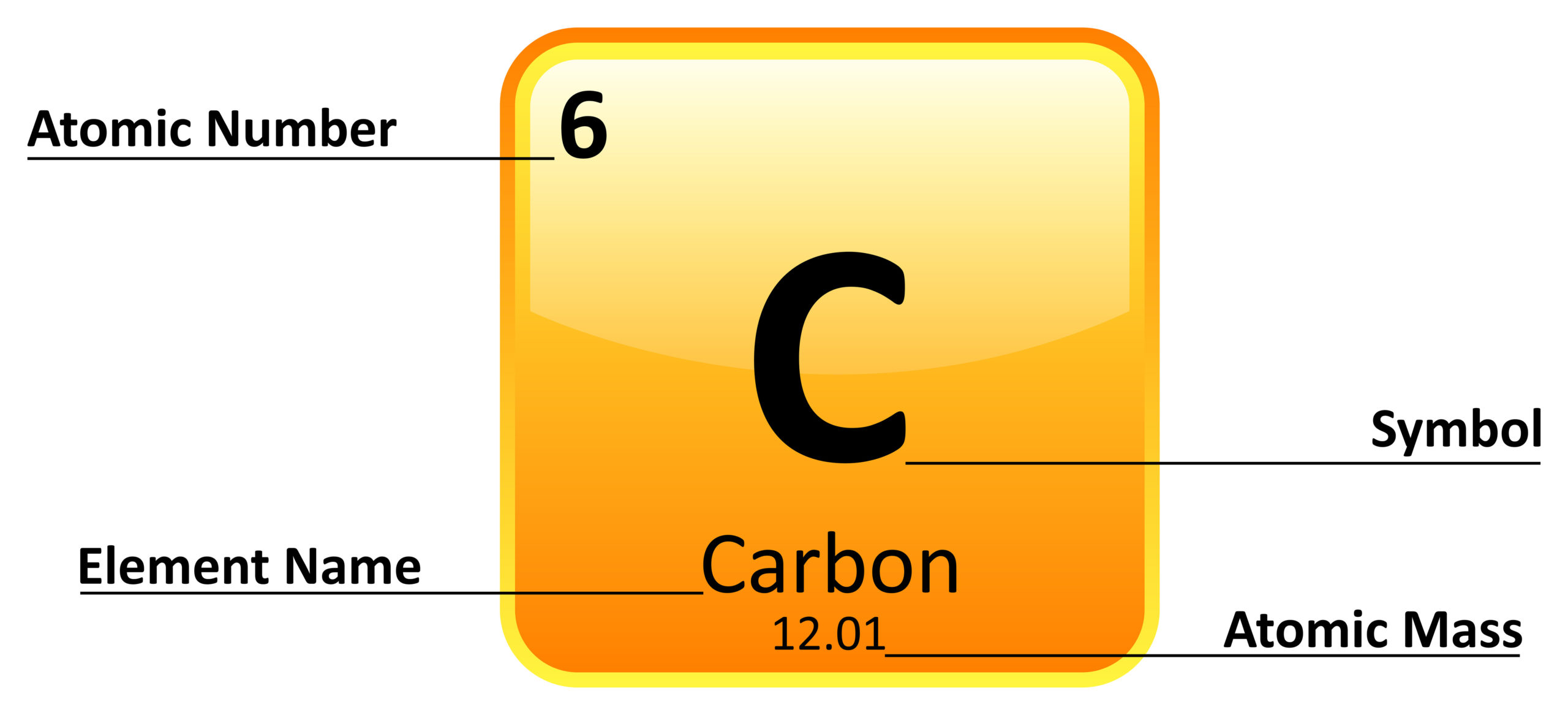 Is this element a metal, non-metal, or metalloid?
Is this element a metal, non-metal, or metalloid?
Non-Metal
What color does litmus paper turn when put on a base? What color does litmus paper turn when put on a base?
The litmus paper turns blue when put on a base, and turns red when put on a acid.
What side on a chemical equation is the reactant side on?
LEFT
Define surface tension?
property of a liquid's surface that allows it to resist an external force (film on liquid's surface that lets things float on surface- water strider)
TRUE OR FALSE: Phosphorus has one less valence electron than Sulfur
True
What is the name of a line that goes left to right called?
Period
What is more acidic 3 or 5.6?
Lower
What does a chemical equation show?
It shows how the reactants rearrange to form the products of the reaction.
What is capillary action, and what is something that uses it everyday?
force that allows water to move upward against gravity, plants move water upward from their roots
Define homogeneous and heterogeneous mixtures
Homogenous mixture looks the same throughout (steel alloy and tea with dissolved sugar), heterogenous mixtures you can see different parts (salad dressing and concrete)