What is the equation used for the ideal gas law?
PV = nRT
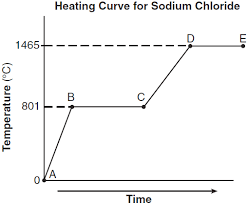
What segment represents the liquid phase? (i.e. A-B, B-C, C-D, or D-E)
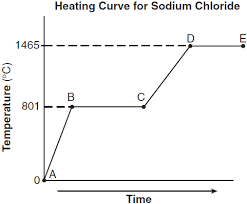
Liquid = C-D
Which element on the periodic table has the highest electronegativity?
Fluorine (F)
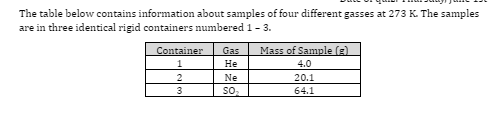
The average speed of the gas particles is
greatest in container 1
greatest in container 2
greatest in container 3
the same in container 1, 2, and 3
greatest in container 1
The human brain is _____ % water (within 5% gets points)
78
A gas has an initial temperature of 20 K and then increases to 40 K. What happens to the volume?
Doubles
Which segment represents the condensation point?
(i.e. A-B, B-C, C-D, or D-E)

Condensation point = D-E
(i.e. A-B, B-C, C-D, or D-E)

What has a larger atomic radius - Cs or Ne?
Cesium (Cs)
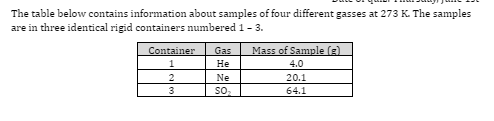
The average kinetic energy of the gas molecules is
greatest in container 1
greatest in container 2
greatest in container 3
the same in container 1, 2, and 3
the same in container 1, 2, and 3
Where does the majority of Earth's oxygen supply come from?
The ocean
A gas a pressure of 2.0 that decreases to 0.5. What happens to the volume?
Quadruples
What is the melting point (hint: look at the Y-axis)

0 C
Which element has the greatest ionization energy?
a) Cs
b) Fe
c) F
d) He
d) He
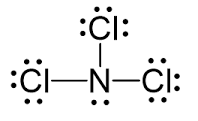
Draw the Lewis Dot Diagram for NCl3
Are there more trees or more stars in the Milky Way galaxy?
Trees
A gas has a starting pressure of 2.0 atm that increases to 4.0 atm. If the initial volume is 3 L, what is the final volume?
1.5 L
What is the boiling point?

100 C

List the following elements in order of smallest to biggest atomic radius:
P, F, He, O
He --> F --> O --> P
What is the VSEPR shape of NCl3?
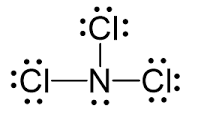
4 domains, 1 lone pair = trigonal pyramidal
The human brain cannot feel ________
A 5L sample of nitrogen gas has a temperature of 300K. If the pressure is 2.0 atm, how many moles of nitrogen gas are there?
PV = nRT
n = PV/RT
n = (2*5)/(0.08206*300) = 0.406 moles
Why is the second dashed segment longer than the first?
More energy required to boil than to melt
Why does F have a larger electronegativity than Ne?
Neon is a noble gas, noble gases do not have electronegativity values
Draw the Lewis Dot Diagram for CO2
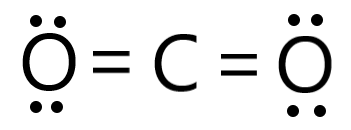
A platypus is a
a) fish
b) reptile
c) amphibian
d) mammal
Mammal