what are the 4 first nations and metis elements on the medicine wheel
What is wind fire rock (earth) water
in 1911 Rutherford ____ charged particles at a very thin foil of gold
what is positively
Cl stands for what element
^
L
what is chlorine
Compound normally consisting of a metal and a non metal have high melting points form crystals dissolve in water and solid at room temperature
what is an ionic compound
alkiali metals and and alkaline earth metals are what two rows on the periodic table.
vertical rows
right beside Each other
what is 1 and 2
a pure substance that is made up of 2 or more elements and are chemically combined ex water
what is a compound
? created the first atomic model in the early 1800s
John dalton
17 of the known elements are ______ they are brittle and not good conductors
what is non-metals
atomic number of Fe or iron
26
what are the three types of mixtures
what is mechanical mixture, suspension, solution.
this is an example of the ? diagram 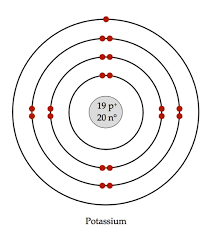
what is Niels Bohr
what 2 elemennts are liquid at room temperature.
bromine (Br) and mercury (Hg).
covalent bonds are a part of which compound
what is molecular
what physical property is described as any solid that can be stretched into a long wire
what is ductility
what is the relative mass in a neutron
what is 1837
what is silicon or Germanium
NaCl, sodium chloride ordinary table salt is an example of what kind of compound
what is ionic
when a substance sticks to other substances this is called?
when it sticks to its self?
what is adhesion
what is cohesion
J.J Thomson experimented with what kind of tube
what is cathode ray tube
helium or helios means what?
what is the sun
row 17 on the periodic table is what
vertical rows
what is halogens