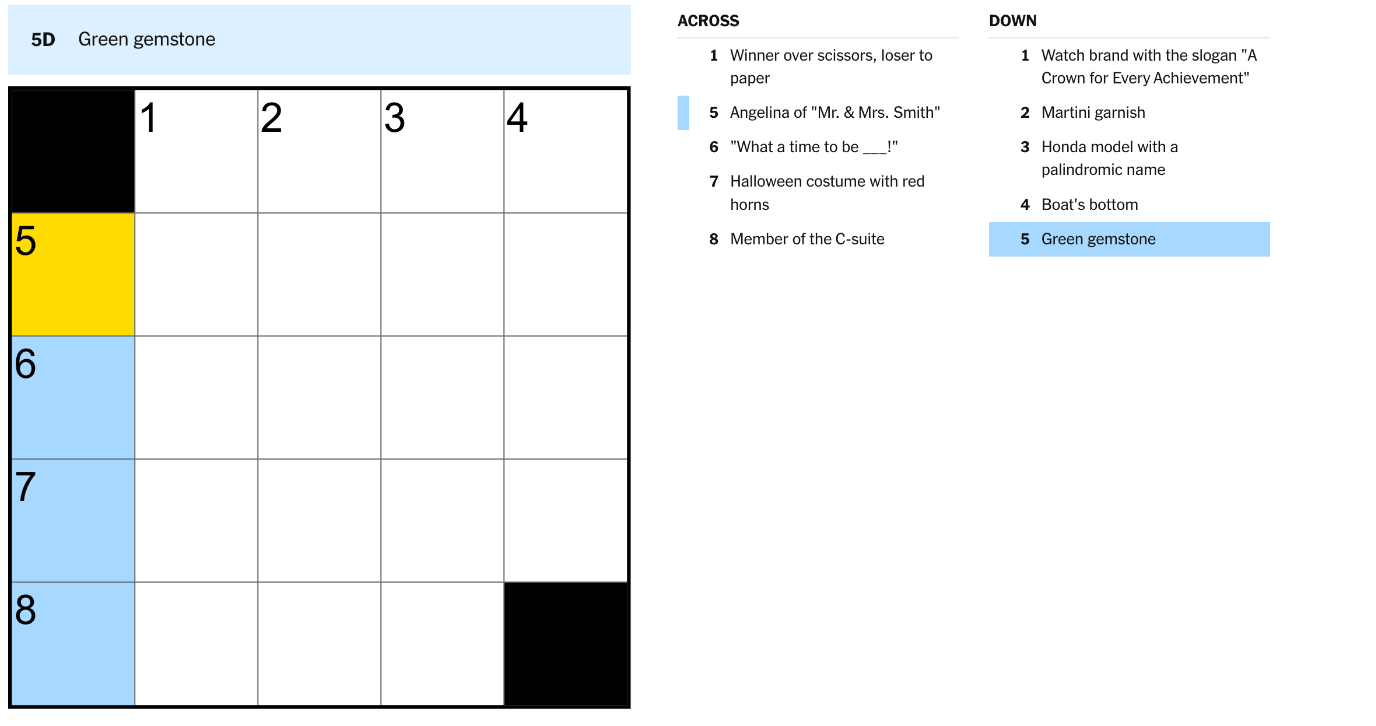
What are the subatomic particles of an atom and what are their charges?
protons: +1
electrons: -1
neutrons: 0
What is the definition of isotopes?
2 forms of the same element (with different numbers of neutrons AKA different mass numbers)
What is the term for a negatively charged ion?
An Anion (ONION)
What family is iodine in? How many valence electrons does iodine have?
Halogens, 7.
In what year was the first email ever sent?
1971
It's atomic number (#protons)
Calculate the atomic number, the number of protons, neutrons, mass number, and number of electrons for the isotope bromine-82 with a charge of -1
atomic number: 35
protons: 35
neutrons: 47
electrons: 35
Draw the Bohr Model for a neutral atom of Aluminum. What charge would it form?
+3
What is the family name of all elements who have two valence electrons?
Alkaline earth metals
What country is this?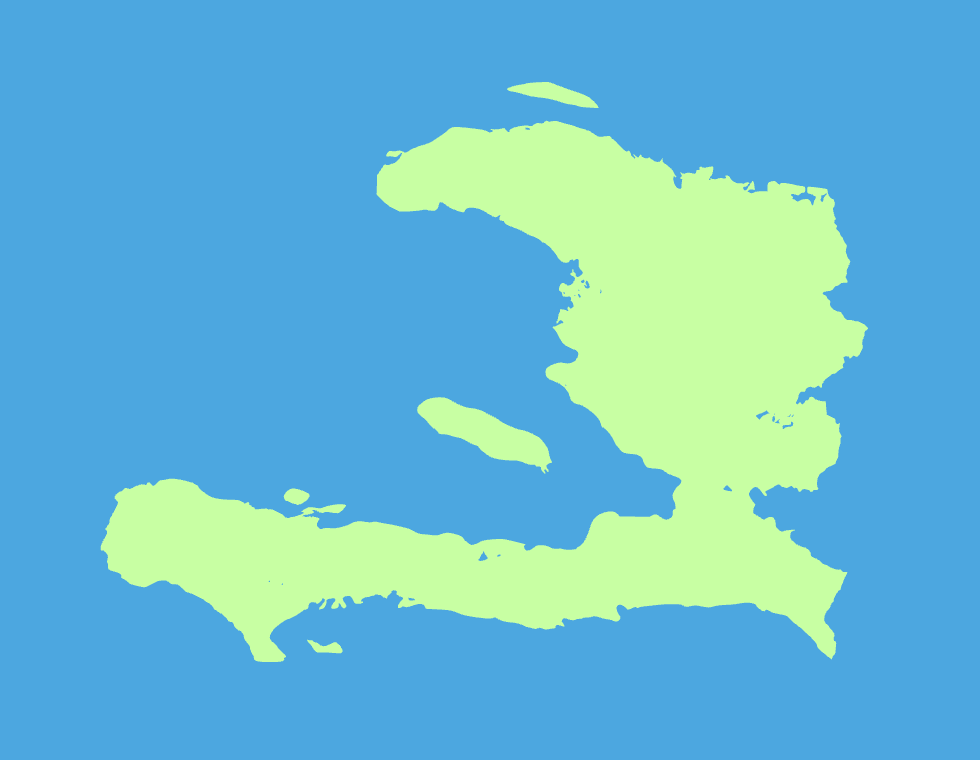
Haiti
What are atoms primarily made up of in terms of its volume?
Empty Space
Write the isotope symbol for vandium-51
51/23V
Determine the numerical charge of a titanium ion with 18 electrons.
+4
Who would be more reactive? Magnesium or Calcium and why?
What is illegal to own as a pet in Switzerland unless you have two of them?
A guinea pig (they get lonely!)
Write the electron configuration for an atom of oxygen. Circle the valence electrons in the configuration.
1s22s22p4
A new element, Element X, has been discovered to have two naturally occurring isotopes. Isotope X-190 has a mass of 189.96 amu and naturally occurs 70.0% of the time. Isotope X-191 has a mass of 191.96 amu. What is the average atomic mass of Element X?
191.360 amu
Write the ionization equation for a neutral atom of Chlorine
Cl + 1e- ----> [ Cl ] -
Write the ionization equation for barium.
Ba --> Ba+2 + 2e-
What is the longest palindrome word? (from literature)
"tattarrattat" by James Joyce
Write the electron configuration for an ion of phosphorous.
1s22s22p63s23p6
The Case of the Missing Isotope Mass
Bromine naturally exists as two primary isotopes. Bromine-79 has a mass of 78.818 amu and a naturally occurring abundance of 50.69%. Using the average atomic mass on the periodic table, determine the mass of bromine's other isotope. Round to 3 decimals
80.953 amu
1. Draw the lewis dot diagram for an atom of sodium. 2. Then draw the lewis dot diagram for an ion of sodium. 3. Finally, write the ionization equation for sodium.
Draw the Lewis Dot Structure for an ion of Sulfur. Then, write an ionization equation for Sulfur.
S + 2e- --> S-2