This is the tool used to measure mass
Triple beam balance or scale
Is DENSITY a physical or chemical property?
Physical property
What mathematical function should you use to find the MASS of an unknown liquid?
(Subtract, add, divide, multiply?)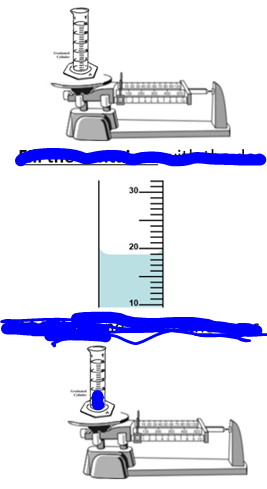
Subtract
An object has a volume of 5 mL and a mass of 30 g. What is the density?
6 g/ml
The volume of a liquid is 5 mL and the mass of the liquid is 25 g. What is the density?
5 g/mL
This is the tool to measure volume
Graduated cylinder
Is this a Physical or Chemical Property?
Physical property
What mathematical function do you use to find the density of an object if you have the volume & mass?
(Subtract, add, divide, multiply?)
Divide
Four boxes with the exact same volume have the following masses:
Blue= 45 g
Red= 15 g
Yellow= 52 g
Which box color has the greatest density?
Yellow
Four boxes with the exact same mass have the following volumes:
Blue= 12 ml
Red= 4 ml
Yellow= 5 ml
Which box color has the greatest density?
Red
g or grams are the units for what physical property?
Mass
How many of the following observations are qualitative?
- Light blue color
- Boiling point of 2,100 degrees
- Rusts when exposed to water
- Dull
- Density of 1.24 g/ml
3
What is the VOLUME of the unknown object?
Volume of Water= 14 ml
Volume of Water + Object=18 ml
4 mL
A cup of gold colored metal beads was measured to have a mass 425 grams. By water displacement, the volume of the beads was calculated to be 48.0 mL. Given the following densities, identify the metal.
Gold: 19.3 g/mL
Copper: 8.86 g/mL
Bronze: 9.87 g/mL
Copper
The volume of a solution was measured in a graduated cylinder to be 45 mL. If the mass of solution is measured to be 60.75 grams, what is the density of the solution?
1.35 g/ml
The units for measuring volume
cm3 or mL
How many of the following observations are quantitative?
- Melting point of 23 degrees
- Light blue color
- Boiling point of 2,100 degrees
- Rusts when exposed to water
- Dull
- Density of 1.24 g/ml
3
What is the volume of the unknown object?
Volume of Water= 28 ml
Volume of Water + Object=43 ml
15 cm3
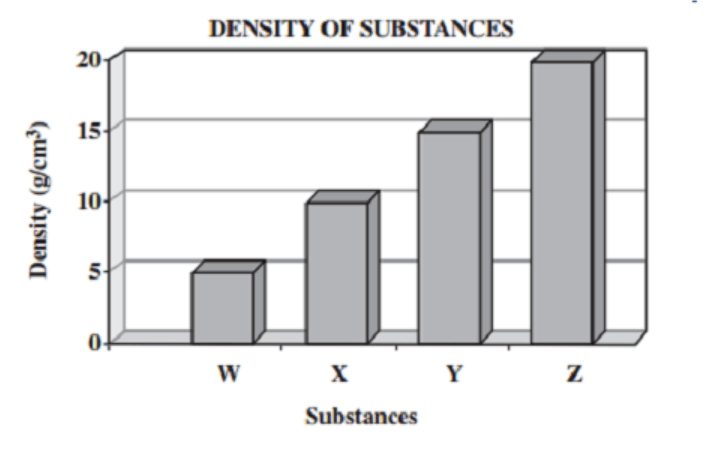 All of these substances have a volume of 2mL. Which one has a mass of about 41 g?
All of these substances have a volume of 2mL. Which one has a mass of about 41 g?
Substance Z
Calculate the density of an unknown object using the data provided below:
Mass of Object=26 g
Volume of Water=14 ml
Volume of Water + Object=18 ml
6.5 g/mL
The units for density
g/mL or g/cm3
How many of the following properties are physical?
- Malleable
- Color
- Reactivity
- Flammability
- Conductivity
- Density
4
What is the mass of the object?
Mass of an empty beaker=20 g
Mass of beaker with object=30 g
10 g
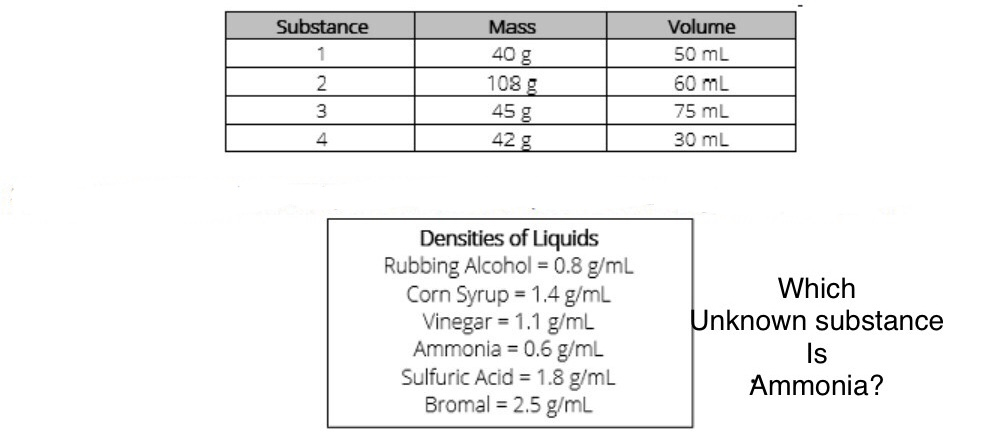
Substance #3
An irregularly shaped stone was lowered into a graduated cylinder holding a volume of water equal to 2 ml. The height of the water rose to 7 ml. If the mass of the stone was 25 g, what was its density?
5 g/mL