Allotropes of carbon include diamond, grahene and this slippery substance
what is graphite?
a pair of electrons that contributes to a molecule's shape but aren't involved in bonding
What is a lone pair?
The property that allows an atom to attract electrons towards itself when forming bonds
What is electronegativity?
The empirical formula of C2H6
What is CH3?
Graphite conducts electricity due to these
what are delocalised electrons?
The shape of something with 3 bonding domains and 1 lone pair such as: 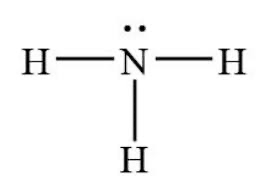
What is trigonal pyramidal?
The molecular formula for ammonia
What is NH3
The number of mol of H atoms in 2 mol of Ca(OH)2
What is 4 mol?
The empirical formula of C12H22O11
What is C12H22O11?
Diamond has a very high boiling point due to these
What are covalent bonds?
The shape of something with 4 electron domains and 2 lone pairs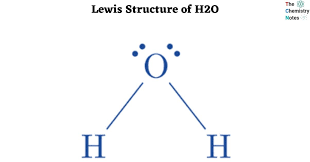
What is bent or v/shaped?
The partial charges on atoms in covalent compounds
What are dipoles?
The molar mass of Cl2
What is 70.0?
The percentage mass of carbon in CH4?
Graphite is slippery because of these weak forces
What are dispersion forces?
Molecules with one or two electron domains always have this shape
What is linear?
The most electronegative element on the periodic table
Fluorine
The units for Molar mass?
g mol-1
If 5.5 mol of methane is combusted, this many mol of water is formed:
CH4 + 2O2 --> CO2 + 2H2O
What is 11 mol?
carbon atoms are arranged in this pattern in graphite
what is hexagonal?
The electron domains around S in this molecule
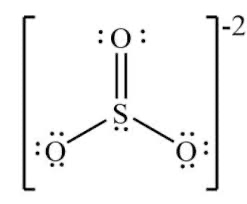
What is 4?
The only Nobel gas that can form an expanded octet
What is Xenon?
The number of mol of CO2 (Mr = 44 g mol-1) in 4.4g?
What is 0.1 mol?
The mass of 2 mol of H2O molecules?
What is 36 g?