Iron and Carbon Dioxide react to form Iron (III) Oxide and carbon monoxide. What is the sum of the coefficients in the balanced equation?
9
Determine the empirical formula for mustard gas, a chemical warfare agent, which has the following composition by mass: 30.2% carbon, 5.07% hydrogen, 44.58% chlorine, and 20.16% sulfur.
C4H8Cl2S
A compound with 2 bonding pairs and one lone pair has what molecular geometry?
Bent
How many bonded electrons are there in the lewis structure for PO43-?
10
What intermolecular forces are present in NCl3?
London Dispersion Forces and Dipole-Dipole
Aluminum reacts with water to produce Aluminum Oxide and Hydrogen Gas. What is the sum of the coefficients of the balanced chemical equation?
8
The empirical formula of a compound if CH2O. Its molar mass is 120.10 g/mol. What is the molecular formula of the compound?
C4H8O4
What is the molecular geometry of PO33-
How many lone pairs are there in the lewis structure for XeF5
16
What intermolecular forces are present in XeCl2?
London Dispersion Forces
Sodium Phosphate reacts with Calcium Chloride to produce Calcium Phosphate and Sodium Chloride. What is the sum of the coefficients of the balanced chemical equation?
12
Find the molecular formula for a compound containing C, H, and O that is 54.5% carbon and 36.3% oxygen by mass. The compound has a molar mass of 176 g/mol.
C8H16O4
SI4 has what molecular geometry?
Seesaw
In the acetylene ion:
H-C≡C:-
What is the hybridization of the carbon on the left? On the right?
sp and sp
Arrange the following by increasing boiling point: CH3CH3, CH3CH2Cl, CH3CH2OH, and CH4
CH4 < CH3CH3 < CH3CH2Cl < CH3CH2OH
In the reaction, where magnesium oxide reacts with water to produce magnesium hydroxide. What mass of magnesium oxide is needed to produce 264g of magnesium hydroxide?
182g MgO
Burning 11.2 mL of a gas known to contain only carbon and hydrogen, we obtained 44.0 mg CO2 and 0.0270 g H2O. Find the empirical formula of the gas.
CH3
What is the molecular geometry of IF5
Square Pyramidal
How many pi bonds are in this molecule? How many sigma bonds?

4 pi bonds, 16 sigma bonds
Arrange the following by decreasing vapor pressure: CH3Cl, CH4, CH3CH2OH
CH4 > CH3Cl > CH3CH2OH
Barium chloride reacts with Sodium Phosphate to produce Barium Phosphate and Sodium Chloride. What mass of barium chloride is needed to react completely with 46.8g of sodium phosphate?
89.2 g BaCl2
A tartaric acid sample containing C, H, and O and weighing 12.01 grams produced 14.08 grams of CO2 and 4.32 grams of H2O under combustion analysis. What is the empirical formula?
C2H3O3
What is the molecular geometry of XeOF2?
T-shaped
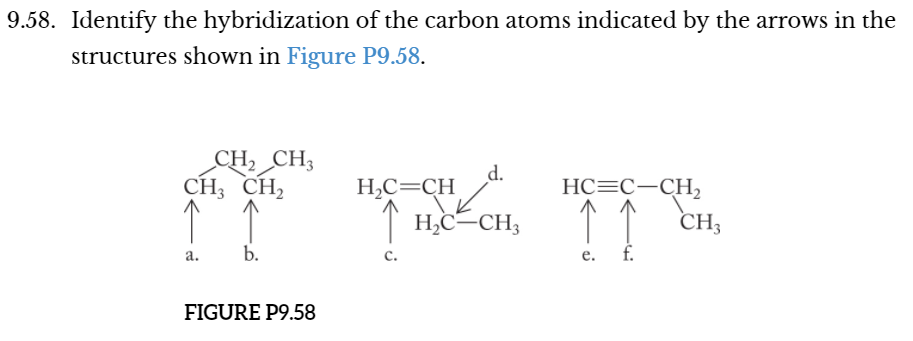
a. sp3 e. sp
b. sp3 f. sp
c. sp2
d. sp3
Which of the following is NOT true?
a) Sulfur tetrafluoride, SF4, is a polar molecule with seesaw molecular geometry.
b) Bromine pentafluoride, BrF5, is a nonpolar molecule with trigonal bipyramidal molecular geometry.
c) CH2Cl2 is a polar molecule with tetrahedral geometry.
d) Boron trifluoride, BF3, is a nonpolar molecule with trigonal planar geometry.
e) Dimethyl ether, CH3OCH3, is a polar molecule.
b) Bromine pentafluoride, BrF5, is a nonpolar molecule with trigonal bipyramidal molecular geometry.