The shape of 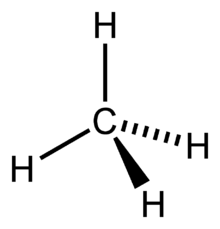
What is tetrahedral
The number of bonds in the molecule CH4.
What is 4?
The angle between atoms in a linear molecule.
What is 180?
The acronym VSEPR stands for this.
What is Valence Shell Electron Pair Repulsion?
What shape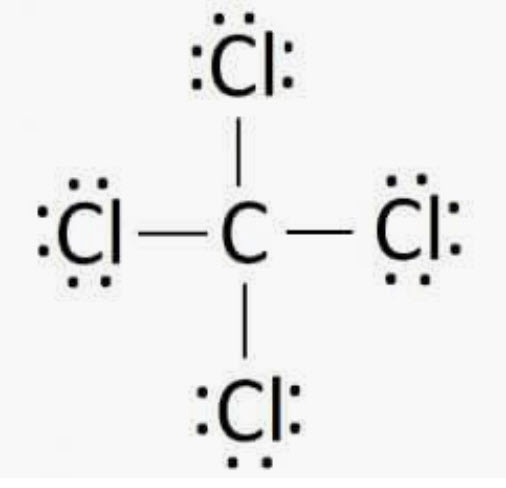
What is tetrahedral
The shape of HCN. 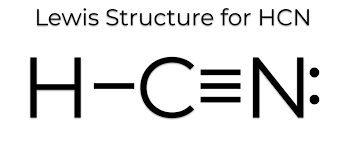
What is linear?
The number of lone pairs in HBr.
What is 3?
The angle between atoms in a tetrahedral molecule.
What is 109?
The reason for a molecule to have a bent shape.
What is lone pairs?
What shape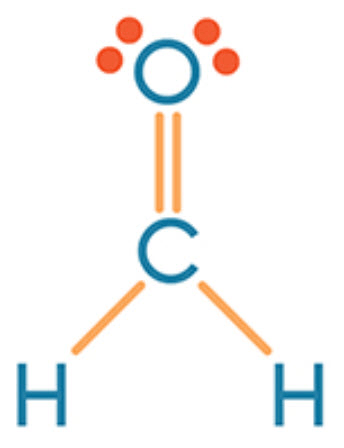
What is trigonal planar
The shape of NH3 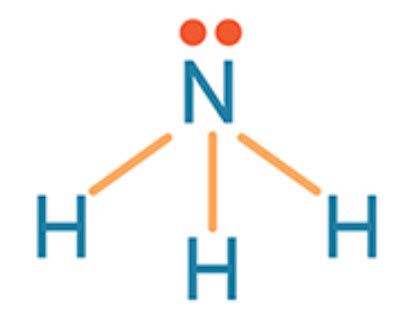
What is trigonal pyramidal?
The types of bond(s) in CH2O
What is single and double?
The angle between atoms in the molecule NH3.
What is 107.5 or 108?
What causes VSEPR shapes.
What is electron repulsion?
What shape 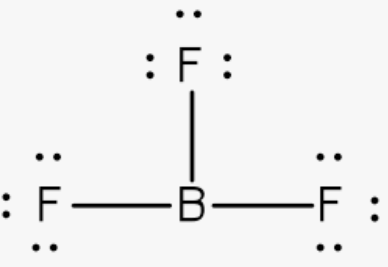
What is trigonal planar
The shape of H2O. 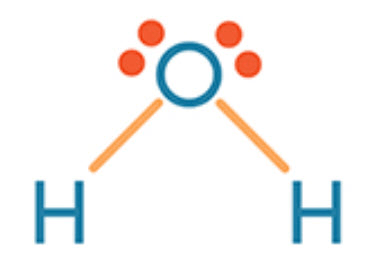
What is bent?
The number of bonds in C2H6
What is 7?
The angle between atoms in the molecule SO2.
What is 120?
Lone pairs and bonds in a molecule tend to be
What is evenly spaced out (spread out)?
What is shape?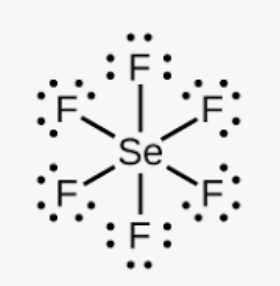
What is octahedral
The shape of CS2 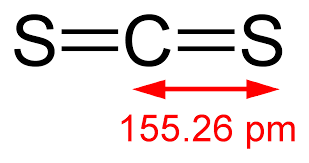
What is linear?
The number of lone pairs in NH3.
What is 1?
The sum of the angles in the molecule CH4.
What is 436?
The shape of VSEPR structures is due to
What is lone pairs & bonds?
What is the shape?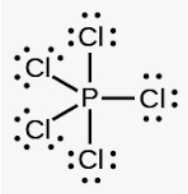
What is trigonal bipryamid