Part of a scientific write-up where you describe why you're doing the experiment.
Purpose
There are 10 of these in 1 centimeter.
millimeters (mm)
In which phase of matter do particles have the most kinetic energy?
gas
What two particles are in the nucleus of an atom? (just name the big particles - not the little things those are made of).
neutrons & protons
Something you might include in a Results section.
Observations, graphs, tables, calculations, data, etc.
Basic metric unit of volume
liter (L)
In which phase of matter are the particles closest together (in all but water)?
solid
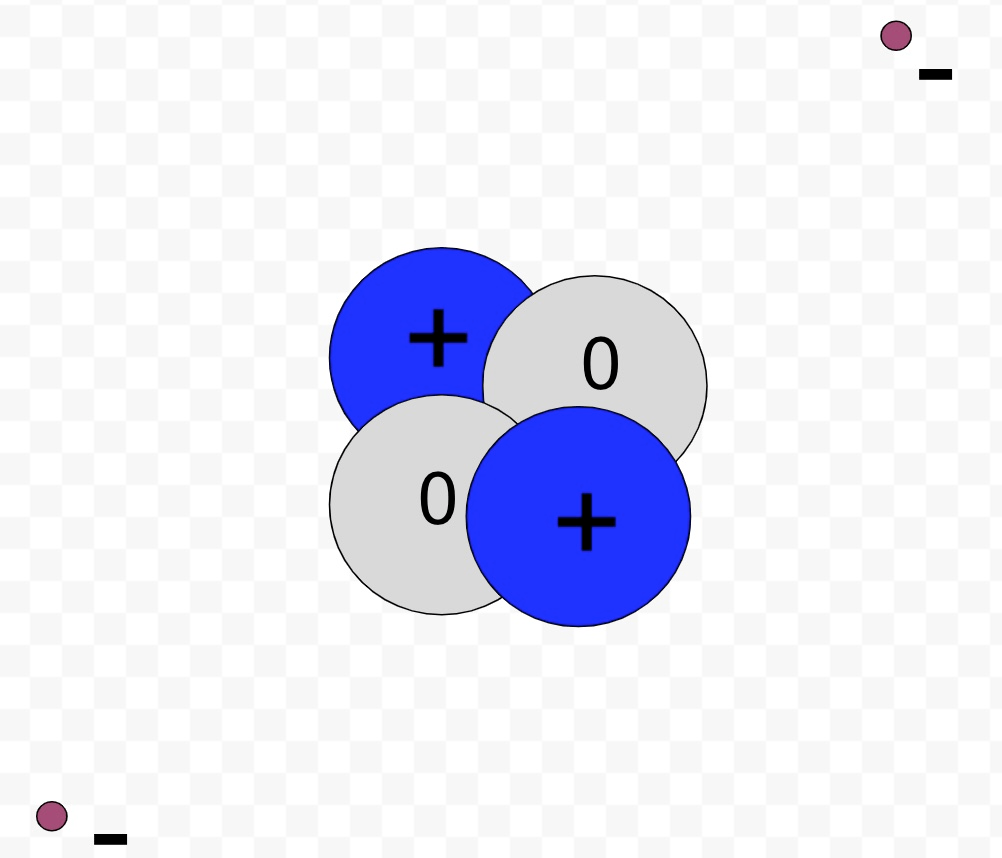
Draw a sketch of a helium atom: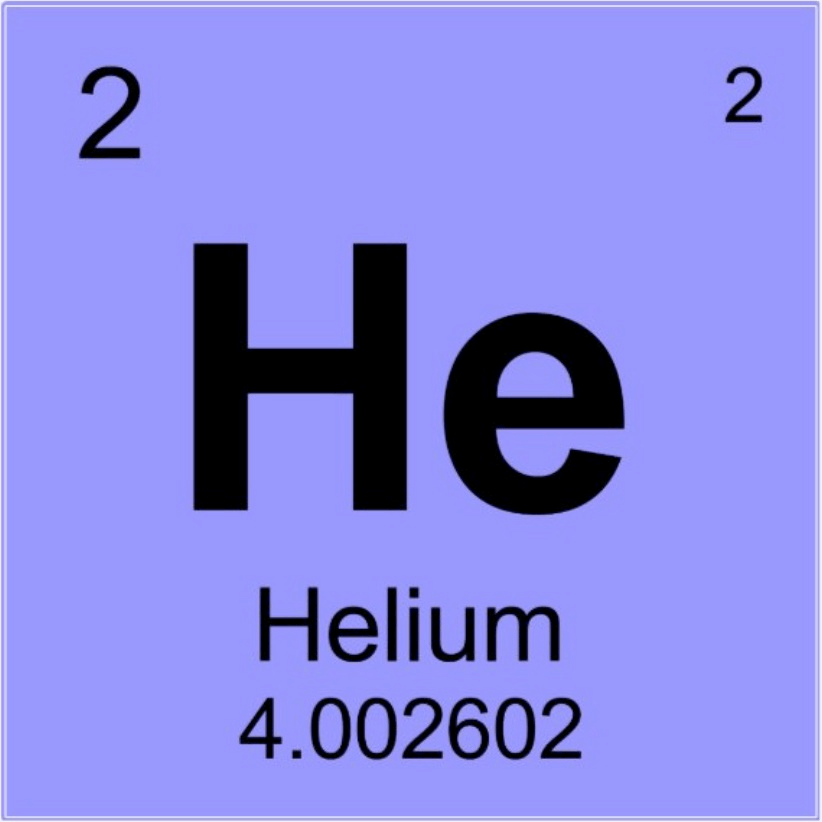
Part of a write-up where you describe the steps you'll take to complete the experiment.
Procedure
Basic metric unit of mass
gram (g)
What do we call it when a gas turns into a liquid?
Condensation
How many neutrons does an average fluorine atom have?

10
The kind of graph we made to show Grow Dinosaur growth over time.
Line graph
What is the density of something that will sink in water?
greater than 1.00g/mL
What word do we use to describe how solid carbon dioxide (dry ice) changes directly from a solid to a gas?
Sublimation
What do all of the elements in a column on the periodic table have in common?
They all have the same number of outer shell electrons (valence electrons).
The name for the vertical lines bracketing these average heights:
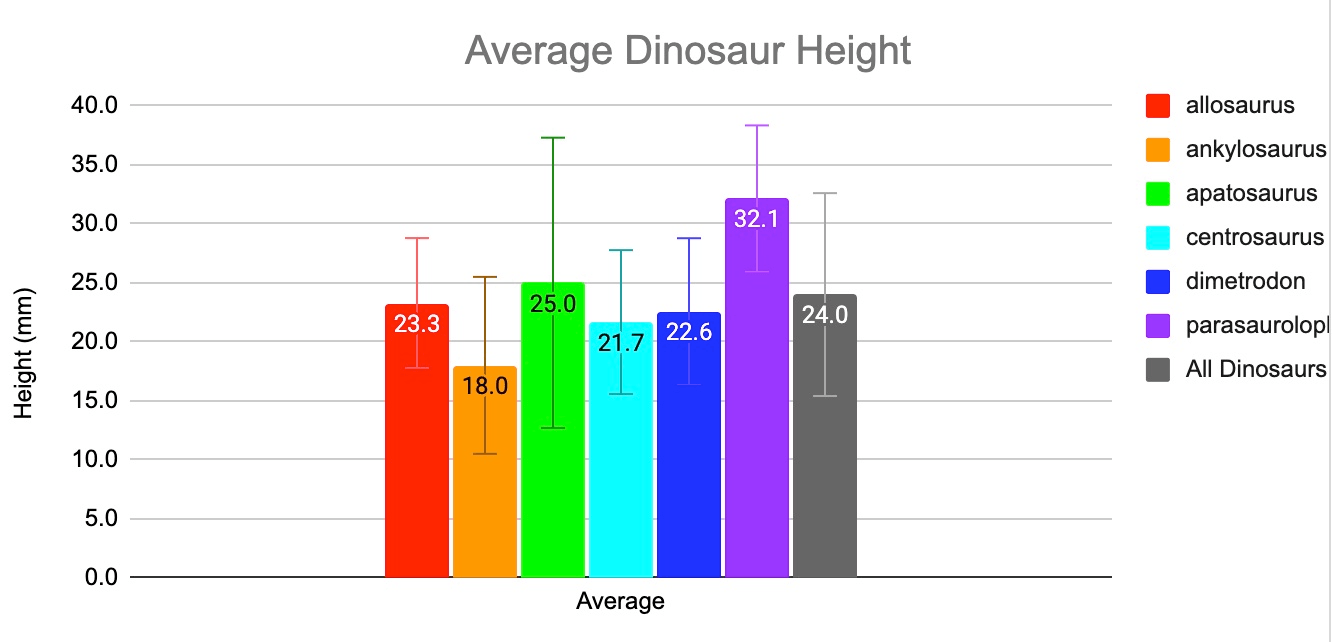
Error bars
How do you calculate an object's density?
mass/volume = density
What's the word that describes water vapor in the air (a gas) going directly to ice crystals (solid) when it's really cold outside?
deposition
What's the name of the "royal" family of elements on the far right side of the periodic table that doesn't want to interact with other atoms?
Nobel Gases