Matter is defined as something that has ______ and takes up space.
Mass
Name the units used for Volume.
mL or cm3
Is boiling point a physical or chemical property?
Physical Property
Homogeneous or Heterogeneous:
A bowl of cereal.
Heterogeneous
Endothermic
 All matter is made tiny units called ______
All matter is made tiny units called ______
Atoms
If an object's mass is 200g, and it's volume is 50mL, what is the object's density?
4 g/mL
Flammability, combustion, and oxidation are examples of _________ properties
Homogeneous or Heterogeneous:
Vegetable soup.
Heterogeneous
Energy is defined as the ability to do _______.
Work
Which of these is a molecule?
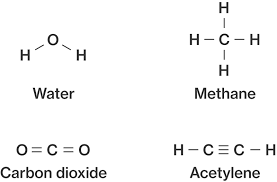
All of them.
Corn syrup has a density of 1.33 g/mL.
Vegetable oil has a density of 0.91 g/mL.
If placed in the same container, which will float on top of the other?
Vegetable oil will float on top.
A type of change in which the form or appearance of matter occurs, but no substances are turned into different substances, is known as a _______ ______
Physical Change
Lemonade is an example of a ___________ mixture.
Homogeneous
Your hydroflasksksksk has an insulating layer between the liquid and the air. This prevents _______ _______ from transfer from the warm air to the liquid.
Thermal Energy
What is the chemical formula for Sulfuric Acid?
H2SO4
Gavin weighs 100 lbs on Earth, and 60 lbs on the moon. How is his mass different on the moon?
No different!
The fact that matter is not created or destroyed in any chemical or physical change is called the Law of __________ __ ____.
Law of Conservation of Mass
A solution is a specific type of mixture involving what form of matter?
Liquid
Thermal energy is always on the move from _______ to _______.
Warmer to Colder
How is a compound different from a molecule?
A compound is two or more different elements. A molecule can have the same element.
Describe how to find the volume of an irregular object.
Water displacement. Drop the object into water and measure the difference in the volume of water.
In order to get this bread, a few things happened:
- You let the dough rise
- You baked the bread in the oven until brown
- You sliced the bread
What types of changes are these events?
Chemical - Dough rising
Chemical - Baking/color change
Physical - Slicing
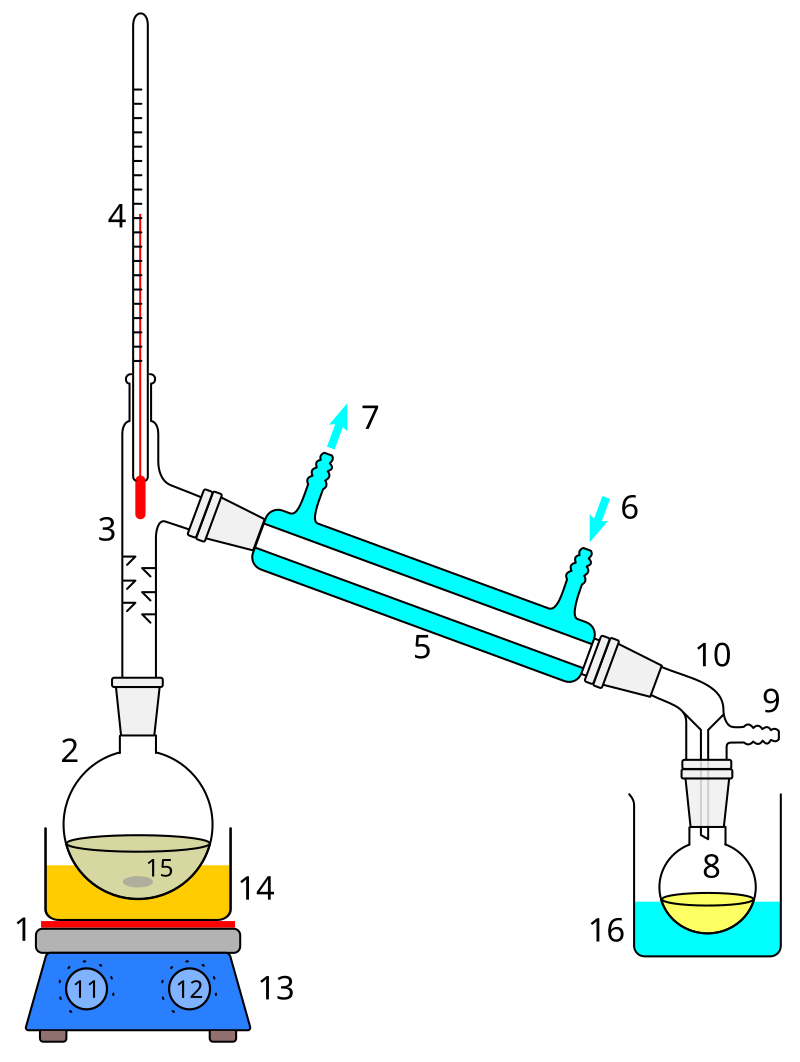 How would you separate a mixture of two liquids with different boiling points?
How would you separate a mixture of two liquids with different boiling points?
Distillation
Name three things that can be produced from a combustion reaction. (such as methane reacting with oxygen)
Heat/Thermal energy, light, carbon dioxide, water vapor