Balance combustion of Hydrogen.
2H2 + O2 --> 2H2O
Name two examples of exothermic reactions.
What are: combustion, condensation of water from vapor, freezing ice, mixing alkali metals in water (sodium, lithium, etc.), digestion, etc.
How many moles are there in 100 grams of Neon?
100 g / 20 g/mol = 5 moles
This element is used in rocket fuel.
This is the longest river in Costa Rica.
What is the Río Grande de Terraba?
Balance combustion of pentane.
C5H12 + 8O2 --> 5CO2 + 6H2O
Name two examples of endothermic reactions.
What are: photosynthesis, melting ice, cooking food, alkane cracking, evaporation
How many grams are in 47 moles of water?
47 moles * 18 g/mol water = 846 g water
Compare and contrast combustion of Hydrogen with combustion of methane (CH4).
Hydrogen combustion produces only water, while combustion of methane produces water and carbon dioxide
Both are exothermic and produce energy
This is Holden's middle name.
What is Thomas?
Balance combination of nitrogen gas and hydrogen gas to make ammonia (NH3); ΔH=−92kJ/mol ammonia
N2 + 3H2 --> 2NH3 +184 kJ
This is the definition of enthalpy.
What is the overall change in energy in a system before and after a chemical reaction?
How many moles are there in 50 g methane (one carbon alkane)?
Methane = CH4
Carbon = 12 g/mol, Hydrogen = 1 g/mol * 4 Hydrogens = 4 g/mol
12 + 4 = 16 g/mol methane
50 g / 16 g/mol = 3.125 mol methane
List one alternative to hydrogen cars that addresses climate change. Explain how it reduces the greenhouse gas emissions of vehicle transportation.
Examples include:
Hybrid cars: braking charges an internal battery, leading to greater fuel efficiency
Electric cars: a battery stores potential energy taken from the electrical grid; if the electricity is powered without greenhouse gasses this eliminates fuel-related carbon emissions
Compressed natural gas: Most common in busses; much more efficient and clean-burning than other fossil fuels, though still produces some greenhouse gases
This is the process used to divide hydrocarbons into different densities.
What is fractional distillation?
Balance the combination of CaCl2 and water to form Ca(OH)2 and HCl, ∆H = +12 kJ/mol HCl
CaCl2 + 2H2O + 24 kJ --> Ca(OH)2 + 2HCl
Balance this equation and indicate if it is endothermic or exothermic.
CaCO3 → CaO+CO2 ΔH=+177.8kJ/mol CaCO3
CaCO3 + 177.8kJ→ CaO+CO2
Endothermic; the energy is a reactant of the equation.
If Lewis's car requires 50 L of diesel every time he refuels, how many moles of diesel does he need to buy?
Diesel = C12H26
1 L diesel = 850 grams
12 * 12 + 1 * 26 = 170 g/mol diesel
50 L * 850 g/L = 42500 g diesel
42500 g / 170 g/mol = 250 mol
Use the chart below to explain the difference between hydrogen and other fuels in kJ/mol vs kJ/gram. Why do you think we see this difference?
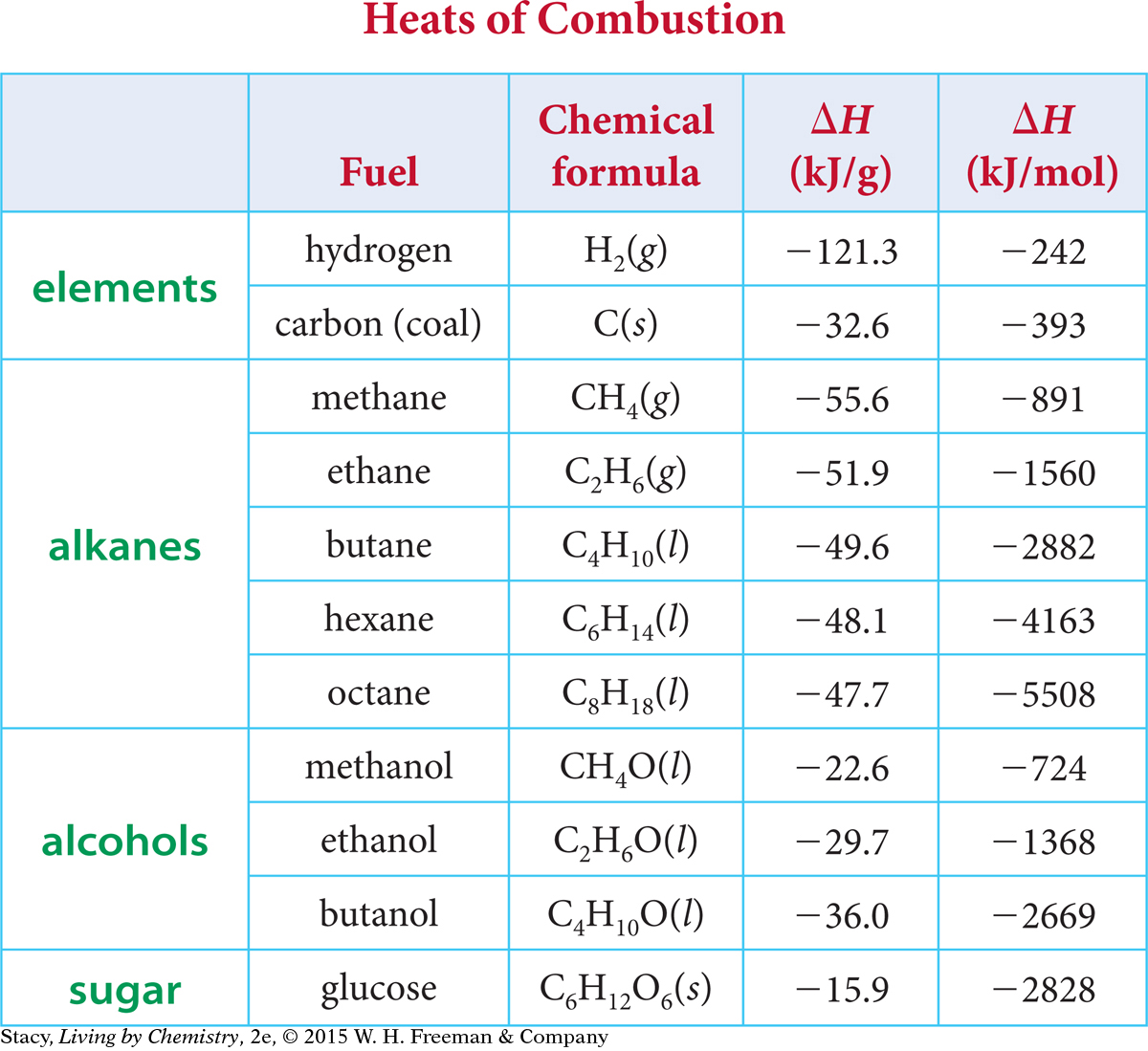
Hydrogen is muuuuuch more efficient in energy produced per gram because it is only 2 g/mol (H2). However, each molecule only has one bond that can be broken to release energy, making it much less energetic per mole.
This is the name of the first Costa Rican president.
Who is José María Castro Madriz?
Balance the combination of NH4OH and H3PO4 to make (NH4)3PO4 and H2O; ∆H = -1112 kJ/mol NH4OH
3NH4OH + H3PO4 --> (NH4)3PO4 + 3H2O +3336 kJ
Balance this equation and say if it is endothermic or exothermic.
C + H2 → C2H2 Δ H = 27 kJ/mol Carbon
2C + H2 + 54 kJ → C2H2
Endothermic
How many moles are there in 1 ton of carbon dioxide?
1 ton = 2,000 lbs
1 lb = 454 g
Molar mass of CO2 = 12 +16*2 = 44 g/mol
1 ton * 2,000 lb / ton * 454 grams / lb = 908,000 grams carbon dioxide
908,000 g carbon dioxide / 44 g/mol = 20,636 moles of carbon dioxide
These are two reasons why Hydrogen cars remain unpopular in most regions of the world.
Answers include:
- High cost of Hydrogen fuel
- Difficult to store / transport
- Lack of infrastructure
-Impracticality of volume of fuel for smaller vehicles
- Use of fossil fuels to produce hydrogen fuel makes them less carbon neutral
This is the name of the founder of Quakerism.
Who is George Fox?