The relative charge of a proton
What is +1?
The name of Group 1 elements
What are alkali metals?
What type of bonding occurs between a metal and a non-metal?
Ionic
What is the relative formula mass (Mr) of carbon dioxide CO2
44
What type of reaction absorbs heat?
Endothermic
The Scientist who proposed the plum pudding model
Who is JJ Thomson
How did Mendeleev predict the properties of elements that had not yet been discovered?
He left gaps
 What is this type of diagram called?
What is this type of diagram called?
Dot and Cross
Calculate the percentage by mass of oxygen in H2O
18/16×100=88.9%.
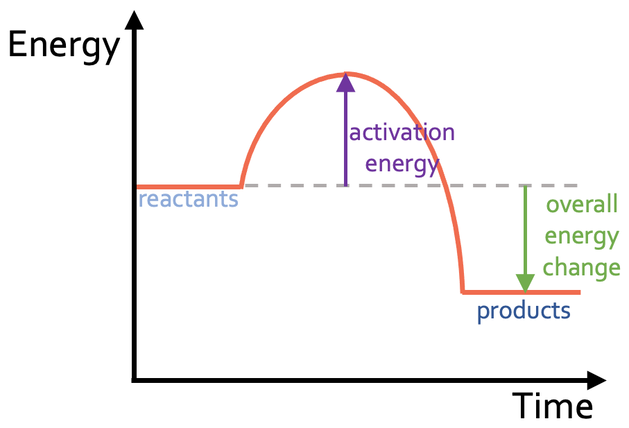
What type of reaction is this
exothermic
Alpha particles prove the existence of "blank" in the atom
What is nucleus?
Why do Group 0 elements have low reactivity?
They have full outer shells
Why are metals good conductors of electricity?
They have delocalized electrons that can move freely.
If 2 moles of hydrogen react with oxygen, how many moles of water are produced?
2 moles.
Define activation energy
The minimum energy required for a reaction to occur.
Calculate the number of neutrons in an isotope of chlorine with a mass number of 35 and an atomic number of 17.
18 neutrons.
Why is potassium more reactive than lithium?
Has more shells- easier to give up its outer electron
Explain why diamond has a high melting point.
Each carbon atom is covalently bonded to four others in a giant structure.
Define the term limiting reactant
The reactant that is completely used up in a reaction, determining the amount of product formed.
How is bond energy related to reaction enthalpy? Write the formula for reaction enthalpy
Reaction enthalpy = Total bond energies of reactants - Total bond energies of products.
why are isotopes chemically identical?
They have the same number of electrons
Predict the product of a reaction between chlorine and potassium bromide solution
Bromine and potassium chloride.
Describe the structure of graphene
Graphene is a single layer of carbon atoms arranged in hexagonal rings;
What is the formula for calculating moles
moles = mass/ Mr
Calculate the energy change if breaking bonds requires 500 kJ/mol and forming bonds releases 700 kJ/mol.
-200 kJ/mol (exothermic reaction).