In which part of the Periodic Table are metals located?
Left
Is sulfur an element or a compound?
Is sulfur trioxide an element or a compound?
Sulfur - element
Sulfur trioxide - compound
What are the three sub-atomic particles?
Protons, electrons, neutrons
iron + oxygen --> rust
What is the chemical name of rust?
iron oxide
What is the name of the compound formed when iron reacts with sulfur?
Iron sulfide
What is the chemical name of the element with symbol B?
Boron
What is the chemical formula of silicon dioxide?
SiO2
What is the charge of the nucleus of an atom?
Positive charge
What is/are required for rusting to happen?
Water and oxygen
What is the salt formed from the reaction between magnesium and sulfuric acid?
Name the element that is located in Group 1, Period 2.
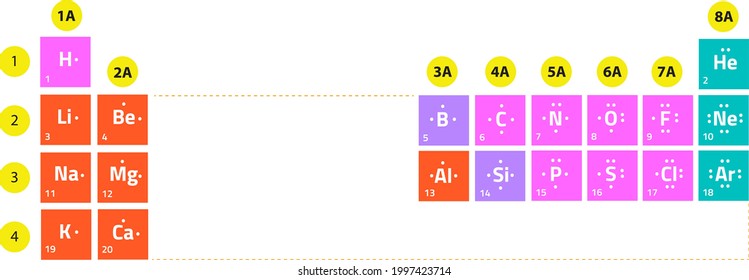
Lithium
Name a compound that contains sodium, carbon and oxygen.
Name a compound that contains potassium, phosphorus and oxygen.
Sodium carbonate
Potassium phosphate
Why does an atom have no charge?
The number of protons and electrons are the same, they balance each other out
What are the products formed when potassium reacts with water?
potassium hydroxide and hydrogen
Write the word equation of the reaction between hydrochloric acid and copper.
hydrochloric acid + copper --> copper chloride + hydrogen
Name one metal that is located in the same period as sodium.

Magnesium / Aluminium
Classify the following into elements and compounds.

Elements: B and D
Compounds: A and C
How are the individual atoms held together?
By electrostatic attraction between the positively charged protons and the negatively charged electrons.
Write down the word equation of the reaction between magnesium and nitric acid.
magnesium + nitric acid --> magnesium nitrate + hydrogen
Metal A reacts vigorously with water to produce hydroxide.
Metal B do not react with cold water but only reacts with hot steam.
Metal C reacts slowly with water to produce hydroxide.
Arrange these metals in order of their reactivity, starting from the most reactive.
Metal A (most reactive), Metal C, Metal B (least reactive)
Where does neon located in the Periodic Table?
Is neon a metal or a non-metal?

Group 8, Period 2
Non-metal
What are the numbers of atoms of each element in H2SO4?
List two differences between the structure of fluorine and that of chlorine.
Fluorine has less protons / electrons / neutrons
What is the gas produced in the reaction of metals with acid?
How could you test this gas?
Hydrogen
If hydrogen is present, a lighted splint will burn with squeaky pop.
Which metal is the most reactive? Explain your answer.
