Which of the following is better at conduction of thermal energy: Water or Air?
Water
Are metals thermal insulators or thermal conductors?
Thermal Conductors
The energy required to heat up 1kg of a substance by 1 degree Celsius
What is specific heat capacity?
The energy required to completely change the state of 1kg of a substance from solid to liquid, without a change in temperature
What is specific latent heat of fusion?
What is internal energy?
Particles gain kinetic energy from a heat source and collide with other particles in the substance, transferring energy to them. Over time, all particles in the substance will gain kinetic energy.
Will a snowman melt faster or slower if it is wearing a thick coat and a woolly hat?
It will melt slower.
J / kg oC
What the units for specific heat capacity?
J / kg
What are the units for Specific Latent Heat?
Explain what is happening to the internal energy of the substance in section 3 of the graph.
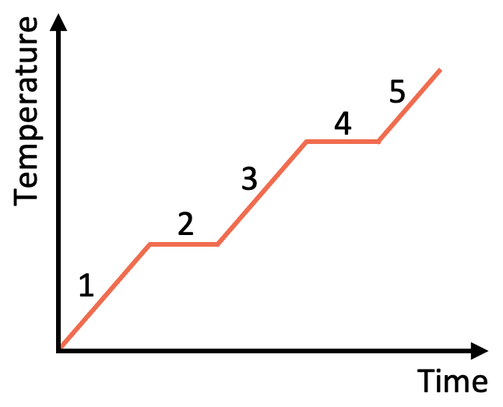
Internal energy increasing
The temperature is rising, so the kinetic energy is increasing.
Internal energy is the sum of potential and kinetic energies of all the particles in a substance.
They have delocalised electrons which can transfer energy through the material
Why are metals (or graphite) good thermal conductors?
Rate of cooling = 13/15 = 0.867 degrees per min
Which substance has the greater specific heat capacity: Ice or Liquid Water?
Liquid Water
For water, which is greater, the specific latent heat of fusion or vaporisation?
Specific Latent Heat of vaporisation
Explain fully why an ice cream melting in the sun is increasing its internal energy, even though its temperature doesn't change.
The potential energy is increasing and the kinetic energy remains constant. The internal energy is the sum of potential and kinetic energy for all particles in a substance. Therefore internal energy is increasing.
Two houses with identical size and shape are next to each other on a street. House A has installed foam insulation between the bricks in the walls. House B has not. Explain which house will heat up faster in the summer.
House B will heat up faster in the summer as the insulation in House A will reduce the rate of transfer of thermal energy from the warm air outside.
State any two control variables needed in an investigation into how thickness of an insulator affects the rate of cooling of hot water.
Mass/volume of water. Type of material. Type of container the water is in. Starting temperature of water. Temperature of the room.
A 300 g copper block is heated over a bunsen burner flame. The temperature rises from 20oC to 63oC. If copper has a SHC of 385 J/kg oC, calculate the thermal energy transferred, in kJ, by the burner flame.
Calculate the energy required to completely evaporate 20g of liquid oxygen, if it has a specific latent heat of vaporisation of 213 kJ/kg.
E = 0.02 x 213000 = 4,260 J = 4.26 kJ
Explain what is happening to the internal energy in Section 2 of the graph.

Internal energy increasing
The temperature is constant but it is changing state, so the potential energy is increasing.
Internal energy is the sum of potential and kinetic energies of all the particles in a substance.
How would you calculate the rate of temperature change at 10 mins for Material 5?
Draw a tangent to the curve at 10 mins. Find the gradient of the tangent.
Gradient = temp change / time = rate of temp change
Two identical beakers are filled with warm water and are both wrapped in the same thickness of the same insulating material. One beaker cools faster than the other. Suggest why this could be.
Different volumes of water used. Different starting temperatures of water. One might have had a lid on top.
A beaker of water is heated by an electric heater. The temperature rises by 31 degrees Celsius when the heater has transferred 87,500 J of energy to the water. Calculate the mass of the water in the beaker. SHC of water = 4200 J/kg oC
m = 87,500 / (4200 x 31)
m = 0.672 kg = 672 g
Calculate the specific latent heat of fusion for carbon dioxide, if 410g of "dry ice" (solid CO2) is completely melted when 75.4 kJ of energy is transferred to it.
L = 184,000 J / kg
Explain why an ice cube will melt faster if it is left outside in the sun, compared with an ice cube which is left on a table indoors for the same period of time.
An ice cube in the sun will gain more thermal energy as the temperature is higher, so the potential energy store of the ice cube will increase faster which means it will change state in shorter period of time.