The three particles which make up an atom are:
Protons, Neutrons and Electrons
For an atom to become an ion, it can ______ or lose electrons.
Gain
Compounds formed when electrons are shared are called ...
Covalent
How many oxygen are present
2 ZnNO3
6
Which particles within an atom add together to give the mass of an atom?
Protons & Neutrons
An atom has an electron configuration of 1s2, 2s2 2p4. This would be an atom of...
oxygen
Complete this word reaction:
Magnesium + Oxygen ----->
Magnesium Oxide
How many Hydrogen are present?
2H2O + H2SO4
6
The atomic number is determined by the number of ...
protons
What would be the electron configuration (in spdf format) for an atom of Aluminium?
1s2, 2s2, 2p6, 3s2, 3p1
Name the following compound:
NO2
Nitrogen Dioxide
Balance this Equation
NaOH + H2SO4 -> Na2SO4 + H2O
2 NaOH + H2SO4 -> Na2SO4 + 2 H2O
Forms of an element where the atoms have a different number of neutrons are called...
Isotopes
What would an atom in Group 7 do to form a stable configuration?
Gain one electron
Give the chemical formula of Potassium Sulfate
K2SO4
Is this Balanced?
2 KMnO4 + 16 HCl ->2 KCl + 2 MnCl2 + 8 H2O + 5 Cl2
Yes
Name this element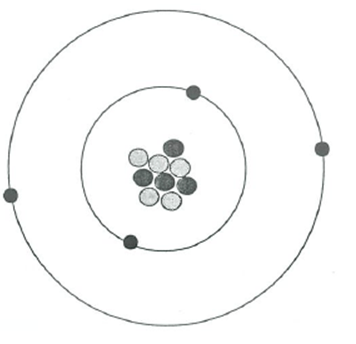
Beryllium
What is the electron configuration (in spdf) of an Oxygen Ion (O2-)
Give the chemical formula of Aluminium Hydroxide
Al(OH)3
Balance this Equation
Fe + Cl2 = FeCl3
2 Fe + 3 Cl2 = 2 FeCl3