Name this molecule: CO2
Carbon dioxide
On the periodic table, in which directions does ionisation energy increase?
To the right and up.
What is one physical property of a chemical substance?
Melting point, Freezing point, Boiling point, Colour, Magnetism, Particle size, Density
What type of bond sees two or more atoms share their electrons?
Covalant
Name an allotrope of carbon.
Diamond, graphite, buckyball, carbon nanotube, graphene etc.
How many years have Aboriginal people inhabited Australia?
65,000 (Anderberg, 2024)
What is Taylor Swift's biggest selling album?
1989
Name this molecule: K2CO3
Potassium carbonate
Which element is the most electronegative on the periodic table?
Fluorine (3.98 on the Pauling scale)
What is one "odd" chemical property you would expect to find in Group I elements?
Highly reactive with water.
In the formation of an ionic bond, what happens to the electrons between the atoms?
Electrons are transferred from one atom to another.
Sodium chloride is an example of what type of chemical structure?
Ionic network
Who is Australia's closest neighbour geographically?
Papua New Guinea (~150km)
In What city is the Australian Formula One Grand Prix held?
Melbourne

What is the chemical formula for sodium nitrate?
NaNO3
On the periodic table, in which directions does electron affinity increase?
To the right and up.
With respect to melting point, which is the odd one out? Ionic network, Covalent network, Covalent molecular network, Metallic structure.
Covalent molecular network
What is the term for the attractive force that holds ions together in an ionic compound?
Electrostatic force
Name this molecular shape.
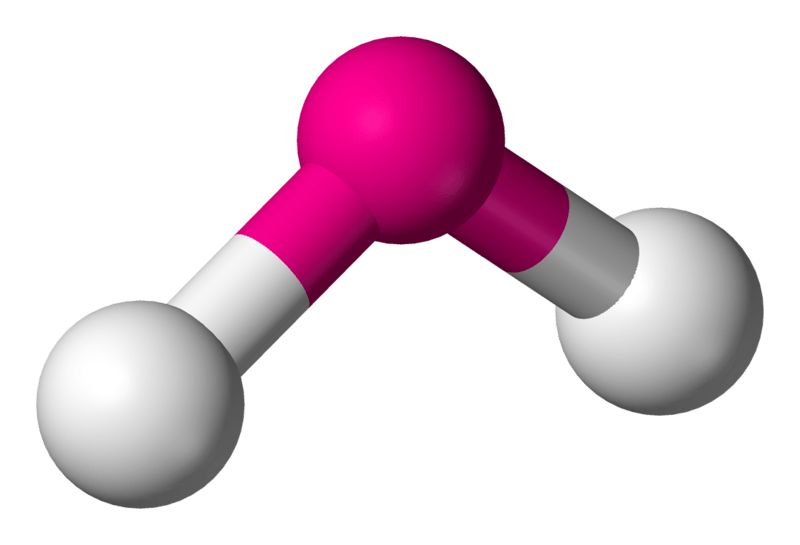
Bent
How many Aboriginal people have competed at the Olympic Games for Australia? (To the nearest 10)
60
What flag is this?

Indonesia
What is the chemical formula for sulfur hexafluoride?
SF6
Explain the trend in atomic radius on the periodic table.
Atomic radius decreases from left to right and increases down a group. This trend is due to increasing nuclear charge across a period and the addition of energy levels down a group.
What physical properties would you expect to find in a substance with short metallic bonds?
High thermal and electrical conductivity, High density, High melting and boiling point.
What type of bond would an electronegativity difference of 1 to 2 produce?
Polar covalent
Name this molecular shape.
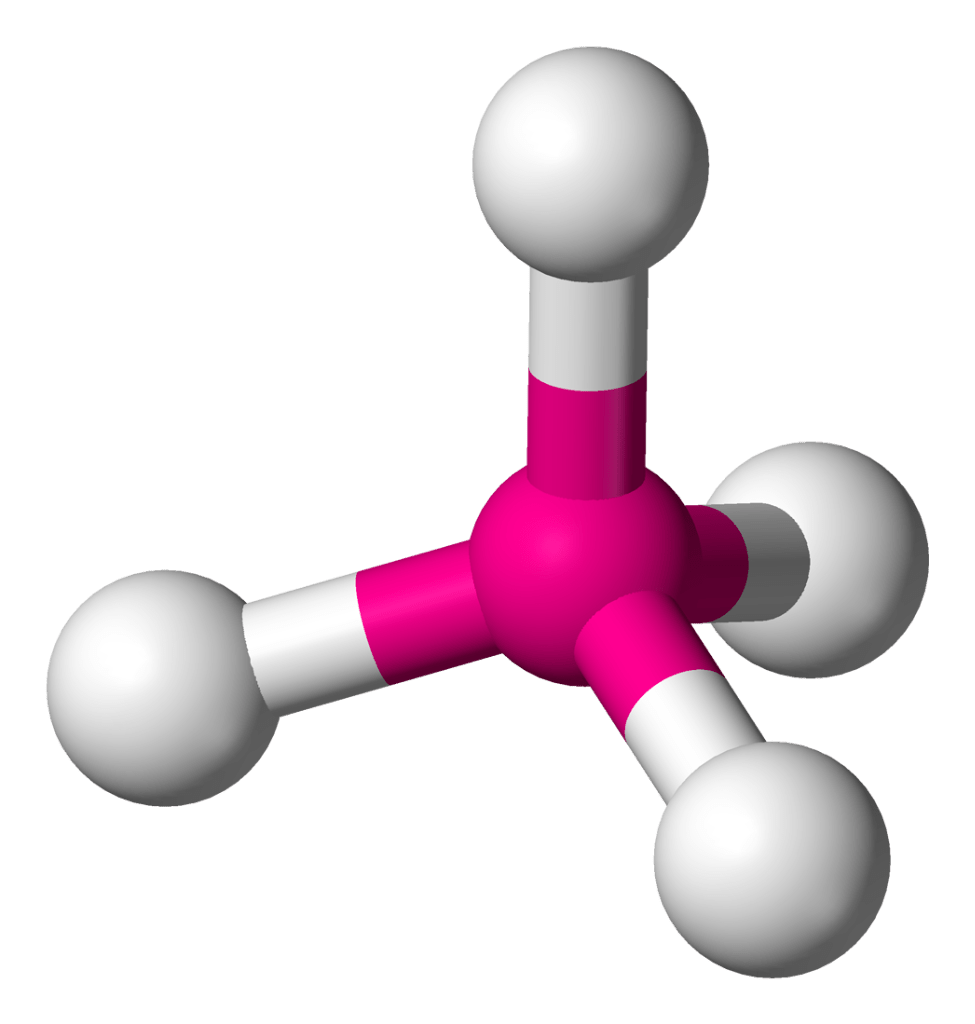
Tetrahedral
Which language, other than English, is most spoken in Australia?
Cantonese/Mandarin
Who is Australia's most successful Olympian?
Emma McKeon (5 gold, 2 silver, 4 bronze)

What is the chemical formula for iron (II) phosphate?
Fe3(PO4)2
Which elements have the highest first ionisation energies?
The Noble Gases
What separation technique is used to remove dissolved salts from a liquid?
Distillation
What is the difference between a polar covalent bond and a nonpolar covalent bond?
Polar covalent bond = electrons are shared unequally between atoms.
Nonpolar covalent bond = the electrons are shared equally between atoms.
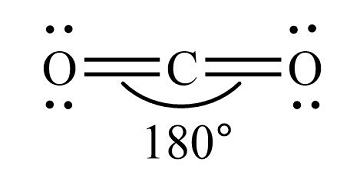
Why is carbon dioxide a linear shape?
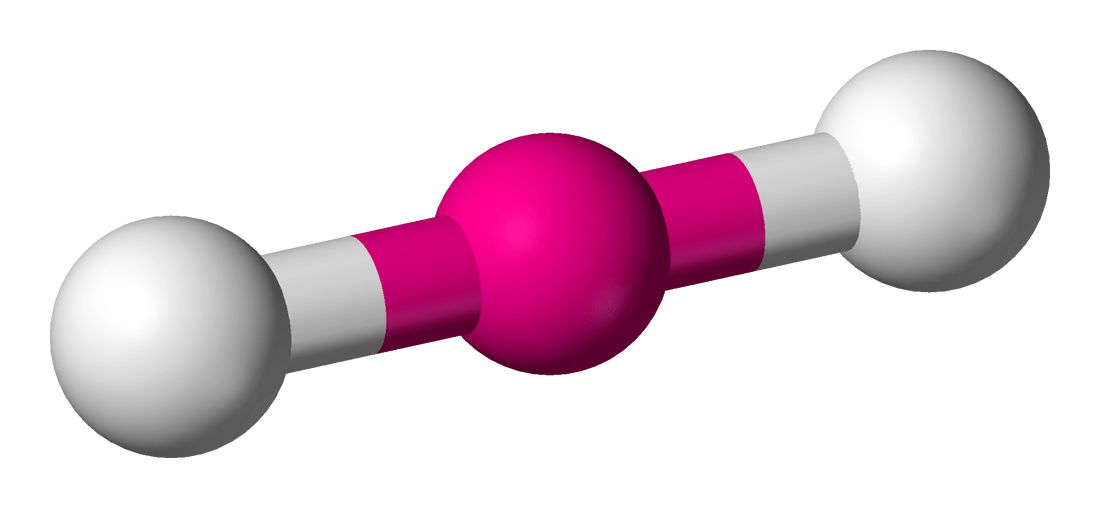
The two lone pairs on each oxygen atom.
What percentage of Australians were born overseas? (to the nearest 5)
29.5%
What is the highest grossing movie of all time?
Avatar ($2.93 billion)