Which of the following states has the weakest bonds between particles?
- Solid
- Liquid
- Gas
- Water
Gas
Which of the statements is FALSE about physical changes?
- Physical changes cause new substances to be formed.
- Physical changes can be easily reversed.
- Physical changes can include a change in state, such as a solid turning into a liquid.
- Physical changes involve a change in shape but not a change in colour or odour.
Physical changes cause new substances to be formed.
If a substance floats on water, this means that it is ________ dense than water.
less
Which of the following substances is an element?
- Water
- Carbon dioxide
- Chlorine
- Salt
Chlorine
Which of the following elements does not have a 1-letter chemical symbol?
- Potassium
- Carbon
- Vanadium
- Beryllium
Beryllium
Which term means “turning from gas to liquid”?
Condensation
Which of the following are examples of physical changes (multiple answers may be chosen here!)
- An ice cream melting
- Mixing together salad ingredients in a bowl
- Baking a cake in the oven
- Boiling water to make steam
- An ice cream melting
- Mixing together salad ingredients in a bowl
- Baking a cake in the oven
- Boiling water to make steam
Which two measurements do you need to know in order to calculate a substance's density?
Its mass (how much of the substance there is)
Its volume (how much space it takes up)
Which of the following substances is a compound?
- Water
- Salt water
- Sea water
- Oxygen
Water (H2O)
Which of the following elements is not a halogen?
A) Chlorine
B) Helium
C) Bromine
D) Iodine
B) Helium
Which of the following statements comparing evaporation to boiling is NOT correct?
- Evaporation occurs slowly, whereas boiling occurs quickly.
- Evaporation requires less heat than boiling.
- Evaporation is a chemical change, whereas boiling is a physical change.
- Evaporation does not involve bubbles, whereas boiling does.
Evaporation is a chemical change, whereas boiling is a physical change.
When making a toasted sandwich, which step involves a chemical change?
- Slicing the loaf of bread.
- Spreading butter and putting a slice of cheese on the bread.
- The bread toasting in the sandwich press.
- The cheese melting when heated up in the sandwich press.
The bread toasting in the sandwich press.
Which one of the following substances is the most dense?
- Gold
- Carbon dioxide
- Wood
- Hydrogen
A) Gold
Which of the following substances is a mixture?
- Hand sanitizer
- Argon
- Water
- Barium chloride
Hand sanitizer
The original Latin name of potassium was kalium, which is why it has the chemical symbol K.
Which of the following elements also has a symbol based on its Latin name?
- Oxygen
- Carbon
- Silver
- Hydrogen
Silver (Latin name was argentum)
Which of these substances turns from a gas to a liquid when cooled down to 100oC?
- Mercury
- Water
- Oxygen
- Aluminium
Water
Mixtures can be separated using physical processes, whereas compounds cannot.
What are three physical processes you might use to separate the substances in a mixture?
Answers may vary
If you were to heat up a liquid, what would happen to the density of that liquid?
- It would increase, becoming more dense.
- It would decrease, becoming less dense.
- It would stay the same.
- It would evaporate.
B. It would decrease, becoming less dense.
Which statement about compounds is true?
- The individual elements in a compound can be separated using phsyical processes, such as boiling.
- The individual elements in a compound are chemically bonded to each other.
- You can see the individual elements which make up a compound.
- A compound is made up of only one type of atom or element.
The individual elements in a compound are chemically bonded to each other.
The elements on the Periodic Table are ordered according to their atomic numbers. For example, Oxygen is the 8th element because it has an atomic number of 8.
Based on this fact, which element on the Periodic Table has an atomic number of 16?
Sulfur
Use the particle model to describe what happens to a substance when heat is applied.
Ensure your answer includes the terms energy and bonds
Answers may vary
Which sentence in the following paragraph describes a chemical property of copper:
Copper is the oldest metal that has been discovered by mankind. The earliest copper artifacts made by humans can be dated back to 8,700 BC (more than 10,000 years ao). Other than gold, copper is the only metal to have a natural colour (brown-red.) All other metals are silver or grey. However, when it reacts with oxygen, copper usually produces a green flame or a green substance, such as copper oxide. In fact, copper oxide is the substance that gives the Statue of Liberty its colour.
Copper is the oldest metal that has been discovered by mankind. The earliest copper artifacts made by humans can be dated back to 8,700 BC (more than 10,000 years ao). Other than gold, copper is the only metal to have a natural colour (brown-red.) All other metals are silver or grey. However, when it reacts with oxygen, copper usually produces a green flame or a green substance, such as copper oxide. In fact, copper oxide is the substance that gives the Statue of Liberty its colour.
On Earth, water has a higher density than oil. This means that water will float on oil.
In space, does water still have a higher density than oil? Explain your answer.
Yes--density is based on mass, not weight. Therefore, density does not change when you go into space.
Fill in the blanks:
In the diagram below, Picture G is the element _________, whereas Picture J is a _________ of two different elements, ________ and __________.
Both of the elements in Picture J are ________ at room temperature.
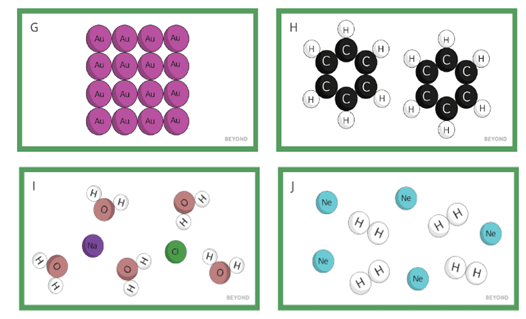
In the diagram below, Picture G is the element Gold, whereas Picture J is a mixture of two different elements, Neon and Hydrogen.
Both of the elements in Picture J are gases at room temperature.
Which of the following chemical symbols does not belong to an element which was named after a famous scientist?
- Rf
- Cm
- Mg
- Bh
Mg (Magnesium)