Which of state of matter has particles with little to no energy?
solid
What is the difference between element and mixtures?
Elements are a type of element
while a mixture will have a physical combination of two or more elements/compounds.
Write the chemical symbol for Chlorine
Cl
What is the MAIN difference between a physical change and a chemical change?
Physical change doesn't form new substances while a chemical change does.
What elements are in the following chemical formula:
NaCl
sodium and chlorine
Identify one shape property for liquids
1. Takes the shape of a container they are placed in
2. Cannot be compressed
Elements only have one type of atom while compounds have two or more different atoms chemically bonded.
What group and period is Nitrogen in?
Group 15, period 2
Describe three evidence of physical change
Change in shape
expansion or contraction
change in state
mixing or dissolving
non-permanent colour change
Carbon dioxide has one carbon atom and two oxygen atoms. Write the chemical formula
CO2
What happens to metals when heat is added?
It expands
Name the three subatomic particles and identify their charge
Proton - positive, Neutron - neutral/zero, Electron - Negative
How many protons does potassium have?
(Hint = atomic number = no. of protons)
19
Name three pieces of evidence that a chemical change has occured.
Permanent colour change
gas produced
precipitate (solid)
change in temperature
light or sound
new substance formed
What is the composition of sodium sulphate?
Na2SO4
What will happen to the size of a blown up balloon if its placed in the freezer and why?
It will shrink because the spaces between particles will contract when heat is removed.
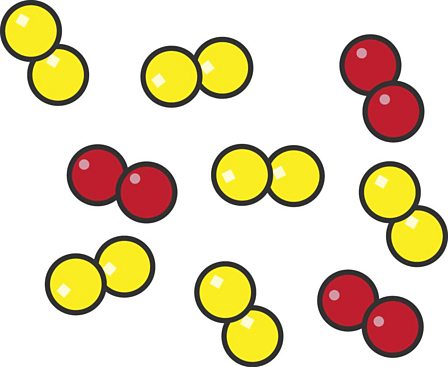
What is the image demonstrating?
mixture of elements
Name a group 1 element.
lithium, sodium, potassium, rubidium, cesium, and francium.
Is this a physical or chemical change? Why?
mixing sand and water.
physical. mixing and reversable.
Identify the reactants and products:
Carbonic acid (H2CO3) is an ingredient in soft drinks. When you open a can of soft drink, a decomposition reaction takes and produces water and carbon dioxide.
Reactants: Carbonic acid, Products: water and carbon dioxide
Define Kinetic Theory
It describes the movement of particles in each state of matter and what happens when heat is added or removed.
Draw a representation of a compound
Two different shapes/sizes used.
Outline the trend of the reactivity as you go down the periodic table
Is this scenario showing a physical or chemical change? Why?
Mixing baking soda and vinegar to produce carbon dioxide gas
chemical. gas produced.
Magnesium sulfate and hydrogen gas are produced when magnesium metal is placed in a solution of sulfuric acid.
magnesium metal + sulfuric acid --> magnesium sulfate + hydrogen gas