What forms in an ionic bond?
A nonmetal and a metal.
The Octet Rule states....
The outer s and p orbitals are completely filled by a total of eight electrons.
What is the chemical name for KCl?
Potassium Chloride
What is the chemical formula for Potassium Iodide?
Kl
When you have this ending, you add the suffix "-ic"
when you have the ending "-ate"
What bonds can conduct electricity and heat?
Metallic and Ionic Bonds (only in liquid form)
Draw Lewis Structure for MgO
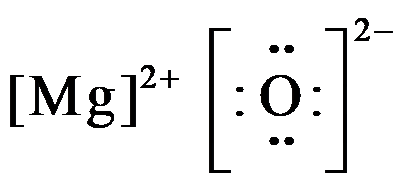
What is the chemical name for PF5?
Phosphorus Pentafluoride
What is the chemical formula of Zinc Sulfate?
ZnSO₄
Name the acid HClO2
Chlorous acid
In an ionic bond, electrons are ____ between ___ of atoms.
Transferred, Valence shells
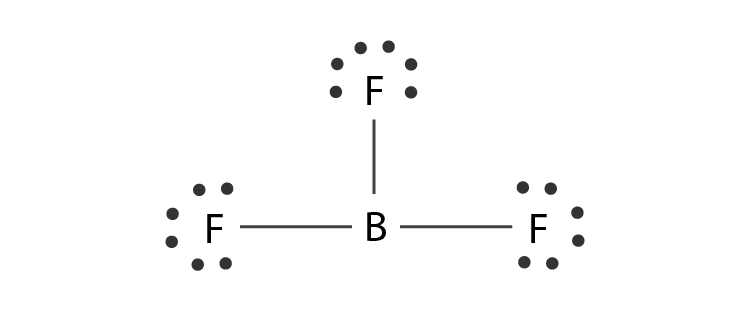
Draw the Lewis Structure for BF3
What is the chemical name for Na2CO3?
Sodium carbonate
What is the chemical formula of Calcium Phosphate
Ca₃(PO₄)₃
Most acids used in the laboratory are either ___ or ___.
Binary acids, oxyacids.
A covalent bond's electronegativity difference is....
<2.0
Draw the Lewis Structure for SCl2
What is the chemical name for Cl2O7?
Dichlorine heptoxide
To find the chemical formula of a compound you have to...
Cross the charges to balance the charges between ions.
Name the acid HBrO₄
Perbromic Acid
What is the texture of a metallic bond?
Usually hard: ductile, malleable, shiny
What is the third step when drawing the Lewis dot structure for covalent compounds?
Determine the total number of valence electrons available for bonding.
What is the chemical name for NH4Cl?
Ammonium chloride
What is the chemical formula of Calcium Perchlorate?
Ca(ClO₄)₂
What is the formula for Perchloric acid?
HClO4
