Mixtures
Complete the sentence.
Liquid is matter with a definite ________ but no definite ________ .
Liquid is matter with a definite volume but no definite shape .
What is the formula for density?
Density = Mass / volume
What group and period is Iron located?
Group 8 and Period 4
Which are the two types of pure substances?
A) elements and solutions
B) matter and mixtures
C) elements and compounds
D) homogeneous and heterogeneous mixtures
C) elements and compounds
Answers will vary.
Which statement accurately compares the arrangement of atoms in the image to their arrangement in solids?
A.) Atoms in the image are close together and move freely, while atoms in solids are far apart and move freely.
B.) Atoms in the image are close together and vibrate in place, while atoms in solids are close together and move freely.
C.) Atoms in the image are far apart and move freely, while atoms in solids are close together and vibrate in place.
C.) Atoms in the image are far apart and move freely, while atoms in solids are close together and vibrate in place.
It is easier to swim in the salt water of the ocean than in a fresh water of a lake.
What statement explains this phenomena?
A) Salt water is less dense than fresh water, so your body will float more easily in sea water.
B) Salt water is more dense than fresh water, so your body will float more easily in sea water.
C) Waves in the ocean make it easier to swim.
D) Your body is less dense in sea water than in fresh water so you float easier.
B) Salt water is more dense than fresh water, so your body will float more easily in sea water.
A scientist is working with an element that is a gas at room temperature and does not react with other elements. Which characteristic BEST describes this element?
A.) Metal
B.) Member of Group 18
C.) Metalloid
D.) Member of Group 2
B.) Member of Group 18
Which of the following substances is a homogeneous mixture?
A) pure water
B) dressing
C) maple syrup
D) sugar crystals
C) maple syrup
Which combination would MOST LIKELY describe a material that is in a solid state?
A.) weak attractive forces and high kinetic energy
B.) weak attractive forces and medium kinetic energy C.) medium attractive forces and high kinetic energy D.) strong attractive forces and low kinetic energy
D.) strong attractive forces and low kinetic energy
Identify the state of matter for each substance.
A - Liquid
B - Gas
C- Solid
If two objects have the same volume, but one has more mass than the other, are the densities of the two objects the same or different?
different
Which two elements on the periodic table are in the same period?
A.) Sn and Rb
B.) F and Cl
C.) K and Ba
D.) Se and Te
A.) Sn and Rb
What type of matter is shown in the photo?
A.) Pure mixture
B.) Pure substance
C.) Heterogeneous mixture
D.) Homogeneous mixture
C.) Heterogeneous mixture
A wooden block is placed in water and floats on the surface. The same block is then placed in an unknown liquid, and it sinks to the bottom of the container. Which statement about the unknown liquid is supported by these observations?
A.) The liquid has a density greater than 1.00 g/cm3. B.) The liquid has a density less than 1.00 g/cm3. C.) The density of the liquid is equal to 1.00 g/cm3. D.) This experiment does not provide information about the density of the unknown liquid.
B.) The liquid has a density less than 1.00 g/cm3.
Which state of matter has the most kinetic energy?
GAS
A rock has a mass of 30g and a volume of 2cm3. What is the density of the rock?
15 g/cm3
Identify element 1 and element 2.
Element 1 - nonmetal
Element 2 - metal
Which statement is NOT true?
A) A pure substance can be separated into elements and compounds by physical methods.
B) A pure substance is made up of only one type of particles.
C) Each substance has unique properties, such as the melting point and boiling point.
D) Elements and compounds are pure substances.
A) A pure substance can be separated into elements and compounds by physical methods.
What physical property helps us the most to determine if an object is a metal, metalloid, or nonmetal?
Kinetic energy increases
List the substances in order from most dense to least dense.
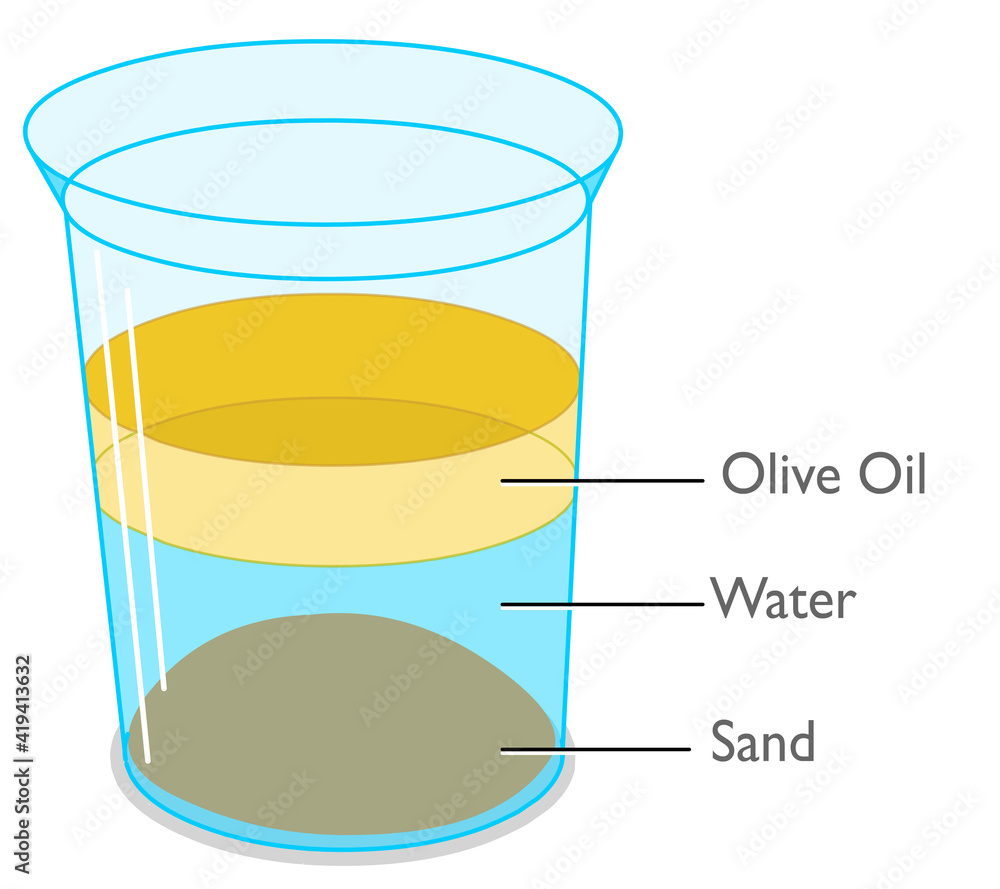
Sand, water, olive oil
Elements that share common characteristics are grouped together on the periodic table. In which part of the table would you MOST LIKELY find an element that is a gas at room temperature?
On the RIGHT (Nonmetals)
Which statement about the mixtures is NOT correct?
A) The substances of a mixture preserve their identity and individual properties.
B) All mixtures are solutions.
C) Mixtures can be separated through physical changes, though some mixtures are hard to separate.
D) Magnets, sieves, filters, and other materials can be used to separate mixtures.
B) All mixtures are solutions.
The mass of a key is 36g. When dropped into a graduated cylinder, the water rose from 30mL to 34mL. What is the density of the key?
9 g/mL