What is Mass Number?
The numbers of protons and neutrons in the nucleus
Calculate the atomic number and mass number of an element that is made up of 4 protons, 5 neutrons, and 4 electrons.
4, 9
How do you calculate the number of protons?
Number of Protons = Atomic Number
What's the first step of drawing Lewis Dot Structures?
a. Locate the valence election number.
b. Give out 1 electron to each side. Once all sides have 1 valence electron- then give another each side.
c. Draw the element's symbol.
What happens to the atom when there are more than 4 valence electrons?
It gains electrons
What is the Atomic Number?
The number of proton in the nucleus?
An atom has a mass number 23 and an atomic number 11. How many protons, electrons, and neutrons are present in the atom?
How to find the number of Neutrons in an isotope?
Subtract the number of protons from the atomic mass of the isotope.
What does a Lewis Dot Diagram ONLY SHOW?
Only shows Valence Electrons.
What happens to the atom when there are less than 4 valence electrons
It loses electrons
What is a Proton?
Atom with the positive charge.
The mass number of X is 23 which contains 15 electrons. What is the atomic mass of X?
23 amu
18 Neutrons
Draw the Lewis Dot structure for BeF2
![]()
How many valence electrons does Magnesium have?
2 Valence Electrons
What is an electron?
Atom with the negative charge.
A radium isotope, on the emission of an
particle gives rise to a new element whose mass number and atomic number will be:
220 and 86
How many neutrons are in Oxygen-17?
9 Neutrons
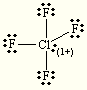
Draw the Lewis Dot Structure for ClF4+
How many valence electrons does Oxygen have?
6 Valence Electrons