This part of an atom has a positive charge.
proton
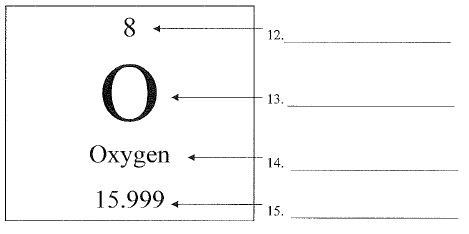
What is number 15 called?
atomic mass
The three main phases or states of matter are.
Solid, liquid, gas
Potassium has this many valence electrons.
1
NH4 has this many atoms.
5
What is the 4 in this formula?
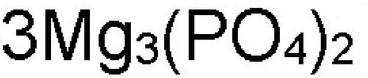
subscript
Calcium
+2
This is an atom that has the same number of protons, neutrons and electrons. It has no charge.
neutral atom
Protons and neutrons are found in this center part of the atom.
nucleus
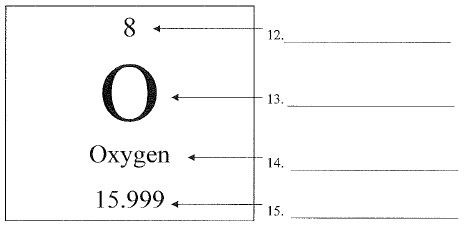
What is number 14 called?
element name or chemical name
When a liquid changes into a gas.
evaporation
Scandium has this many valence electrons.
3
2C2H3N5 has this many atoms.
20
What is the 3 in the following formula?
3H2O
coefficient
Nitrogen
-3
This is an atom that has gained neutrons and now has a higher atomic mass than a neutral atom.
heavy isotope
The part of the atom that has no charge (neutral).
neutron
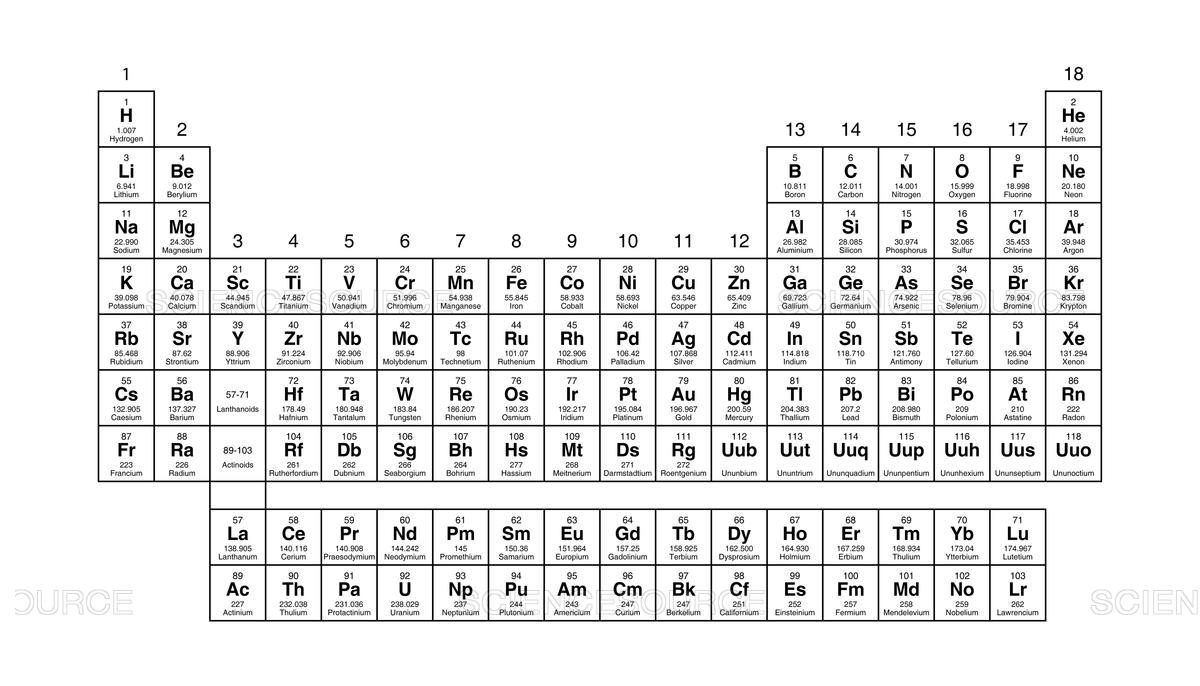 The columns on the Periodic Table are called this.
The columns on the Periodic Table are called this.
groups
When a solid changes to a liquid.
melting
Arsenic has this many valence electrons.
15
2C4H8FCOOH has this many HYDROGEN atoms.
18
What is Mg in this formula?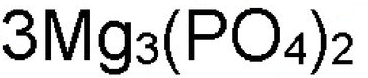
chemical symbol or element symbol
Chlorine
-1
This is an atom that has gained electrons. It now has a negative charge.
anion
These negative parts of the atom are found in levels orbiting around the center.
electrons
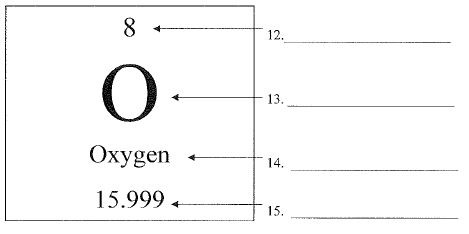
What is number 12 called?
Atomic Number
When a liquid changes into a solid.
freezing or solidifying
Xenon has this many valence electrons.
0 or 18
2S4H5(F2Ti3)3 would have this many atoms.
48
What is between the 4 and the 2 in this formula?
3Ba3(PO4)2
parenthesis or brackets
Titanium
+4
This is an atom that has LOST neutrons and now has a smaller atomic mass than a neutral atom.
light isotope
List how many electrons can fit into all the shells (energy levels) in order from the first shell (level) to the seventh.
2, 8, 8, 18, 18, 32, 32
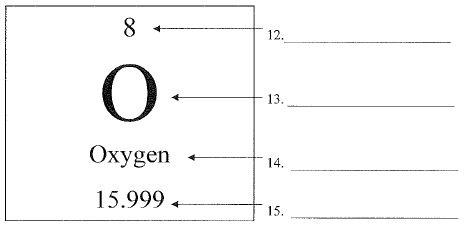
What is number 13 called?
chemical symbol or elemental symbol
When a gas changes into a liquid.
condensation
Boron has this many valence electrons.
3
4(NH4)3PO4 would have this many atoms.
80
What does a coefficient do?
multiplies everything in the formula or tells you how many molecules there are
Radon
0
This is an atom that has LOST electrons and now has more protons than electrons. It has a positive charge.
cation
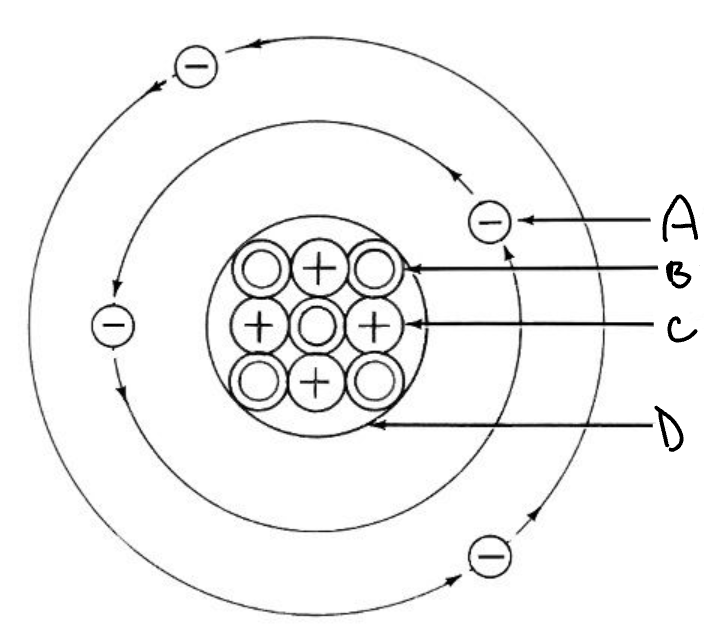 Name all 4 things that the letters are pointing to.
Name all 4 things that the letters are pointing to.
A-electron
B-neutron
C-proton
D-nucleus
You find the number of neutrons in an atom by doing this.
atomic mass - atomic number
The fourth state of matter. Stars are made of this.
Plasma
List all of the "Noble Numbers"
2, 10, 18, 36, 54, 86
3Cr(NH3)6(NO3)3 would have this many NITROGEN atoms.
27
What does a subscript do?
Tells you how many atoms you have of the element symbol that came before it
Chromium
+6
What do we call an atom that does not have equal protons and electrons and has a charge?
ion
Protons, neutrons and electrons are all types of what?
Subatomic particles
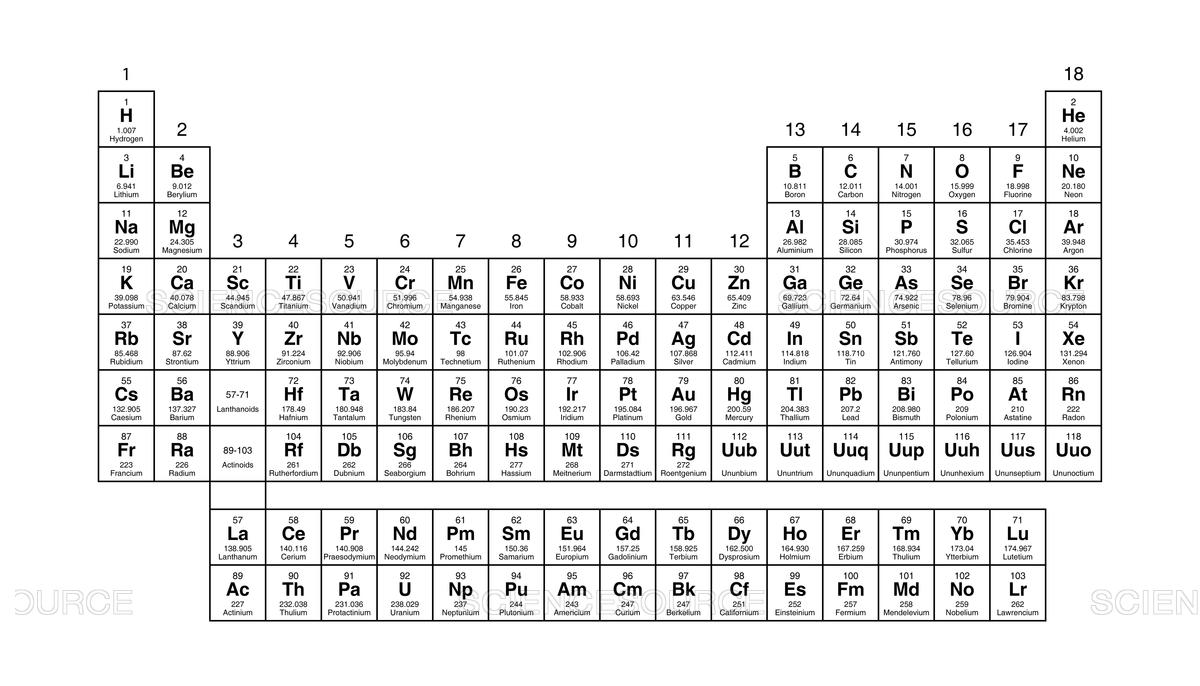 The rows on the Periodic Table are called this.
The rows on the Periodic Table are called this.
periods
When a solid changes directly into a gas.
sublimation
Who created the Periodic Table?
Dmitri Mendeleev
2K4Fe(SCN)6Cr2(SO4)3 has how many total atoms?
80
Connects a smaller set inside a formula. They always come in pairs.
Antimony
-3
What do we call an atom that has more or less neutrons than it is supposed to have which changes its atomic mass?
isotope
What state of matter forms a blob at near absolute zero?
Bose Einstein Condensate